JEE Class main Answered
Question: in a crystalline solid of XY3 cubic closed packed arrangement for its element Y,,,, X occupies.... ? Answer: 33% OCTAHEDRAL VOIDS.
how was this worked out? not the math necessarily,, but the voids occupied by X being OCTAHEDRAL and not TETRAHEDRAL. i don’t understand this part. i’d really appreciate it if someone could walk me through the logic here. thanks.
Asked by astutijoshi | 03 Jun, 2019, 17:12: PM
Given:
XY3 has CCP arrangement for element Y.
atoms in one unit cell of CCP = 4
No. of atoms of X = 4/3
No. of octahedral voids = 4
So, % of octahedral voids filled
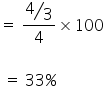
The number of octahedral voids present in lattice is the same as the number of close- packed particles,
whereas, the number of tetrahedral voids is the twise of that number.
Therfore the total number of octahedral voids is 4 and tetrahedral voids is 8 per unit cell.
Answered by Varsha | 04 Jun, 2019, 11:21: AM
JEE main - Chemistry
Asked by astutijoshi | 03 Jun, 2019, 17:12: PM
JEE main - Chemistry
Asked by inbasri224 | 23 Feb, 2019, 23:48: PM
JEE main - Chemistry
Asked by Jagilinkinylu | 29 Dec, 2018, 20:03: PM
