JEE Class main Answered
Please expalin the following

Asked by Balbir | 23 Dec, 2018, 15:50: PM
MgCl2 + 2NaOH → Mg(OH)2 + 2NaCl
No of Millimoles (t0) 10 20 0 0
No of Millimoles (t) 0 0 10
So, [Mg2+] = 10/200 = 1/20
Ksp Mg(OH)2 = S[Mg2+] × S(OH-) = {1/20} × {4 (OH-)2}
2 (OH-) = 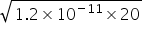 =
= 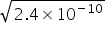 =
= 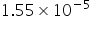
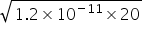 =
= 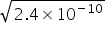 =
= 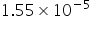
(OH-) = Near to 10-5
Answered by Sumit Chakrapani | 03 Jan, 2019, 03:56: AM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by neerajavuppala1983 | 23 Jul, 2024, 22:49: PM
JEE main - Chemistry
Asked by tanniruv133 | 03 Jul, 2024, 18:50: PM
JEE main - Chemistry
Asked by heyyyyy | 12 Jun, 2024, 19:18: PM
JEE main - Chemistry
Asked by hv5594265 | 12 Jun, 2024, 11:59: AM
JEE main - Chemistry
Asked by rupalibhange1987 | 11 Jun, 2024, 20:00: PM
JEE main - Chemistry
Asked by gajju8493 | 11 Jun, 2024, 15:09: PM
JEE main - Chemistry
Asked by chakrabortymithu041 | 29 May, 2024, 18:45: PM













