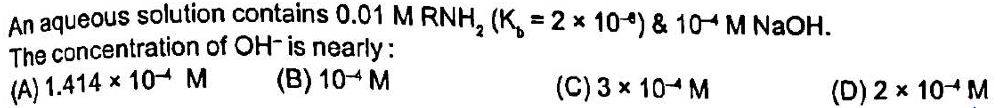JEE Class main Answered
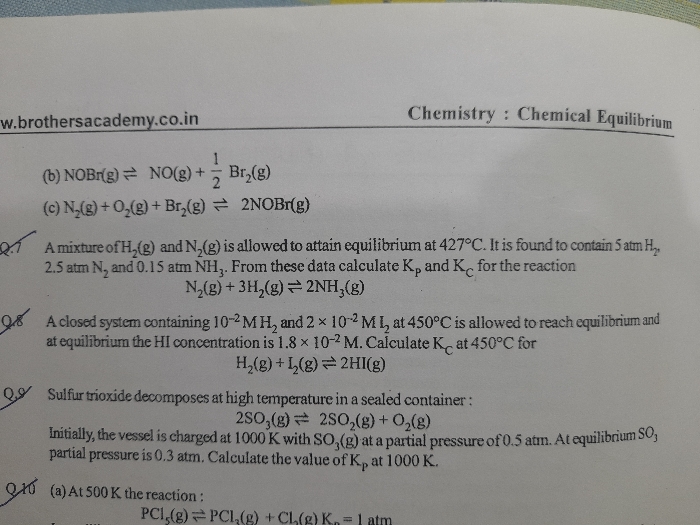
NH2COONH4(s) ⇔ 2NH3(g) + CO2(g)
In a closed vessel at 298K starting with only NH2COONH4(s) an equilibrium is established after sometimes. Then some ammonia is introduced and at the new equilibrium the partial pressure of NH3 equals the total pressure of old equilibrium.
Find (Pt final)/(Pt initial)
Asked by gargchahat2005 | 13 Dec, 2020, 17:16: PM
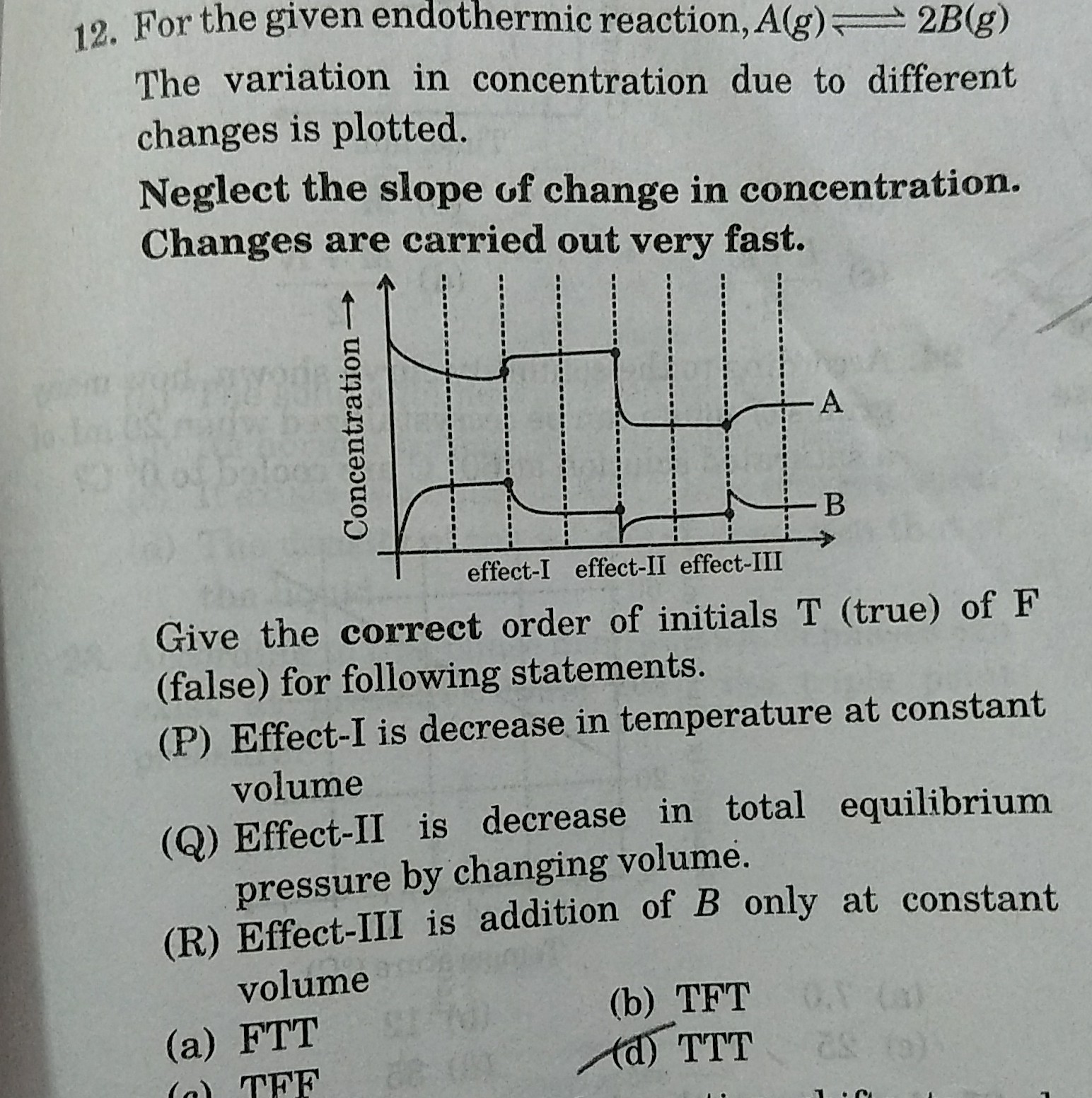
It is a question based on equilibrium.
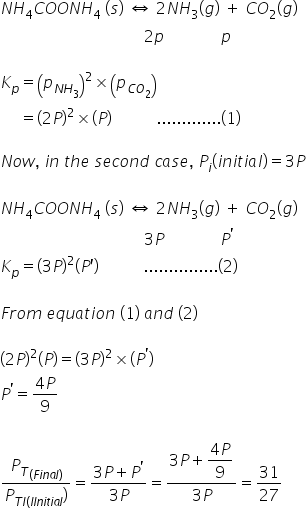
Answered by Ravi | 14 Dec, 2020, 18:15: PM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by mokayesu162 | 26 Nov, 2023, 13:40: PM
JEE main - Chemistry
Asked by kumardevanand11825 | 04 Jun, 2022, 14:07: PM
JEE main - Chemistry
Asked by pritgambhwa | 31 Dec, 2021, 16:36: PM
JEE main - Chemistry
Asked by gargchahat2005 | 13 Dec, 2020, 17:16: PM
JEE main - Chemistry
Asked by Yasharthshankar121 | 29 Jun, 2020, 18:31: PM
JEE main - Chemistry
Asked by rgunasekhar2222 | 26 Jun, 2020, 08:46: AM
JEE main - Chemistry
Asked by monjittaye16 | 23 Apr, 2020, 17:10: PM