JEE Class main Answered
ionisation energy of he+1 is x. find ionisation of energy of li2+
Asked by atwalreet102 | 06 May, 2023, 21:21: PM
Dear Student,
Ionisation energy of He+1 is x.
ZHe = 2, ZLi = 3
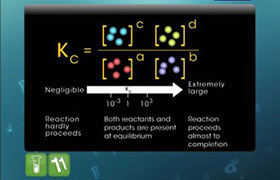
(IE1)He+ / (IE1)Li2+ = (ZHe)2 / (ZLi)2
x / (IE1)Li2+ = 4 / 9
(IE1)Li2+ = x × 9 / 4
Hence, Ionisation energy of Li2+ = 9x/4
Answered by | 09 May, 2023, 12:11: PM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by neerajavuppala1983 | 23 Jul, 2024, 22:49: PM
JEE main - Chemistry
Asked by tanniruv133 | 03 Jul, 2024, 18:50: PM
JEE main - Chemistry
Asked by heyyyyy | 12 Jun, 2024, 19:18: PM
JEE main - Chemistry
Asked by hv5594265 | 12 Jun, 2024, 11:59: AM
JEE main - Chemistry
Asked by rupalibhange1987 | 11 Jun, 2024, 20:00: PM
JEE main - Chemistry
Asked by gajju8493 | 11 Jun, 2024, 15:09: PM
JEE main - Chemistry
Asked by chakrabortymithu041 | 29 May, 2024, 18:45: PM