JEE Class main Answered
In a reversible reaction 2NO2<--K1—K2---->N2O4, the rate of disappearance of NO2 is equal to:(K1 is rate of forward reaction, K2 rate of backward reaction.)
1) 2K1[NO2]2/K2
2) 2K1[NO2]2-2K2[N2O4]
3) 2K1[NO2]2-K2[N2O4]
4) 2(K1-K2)[NO2]
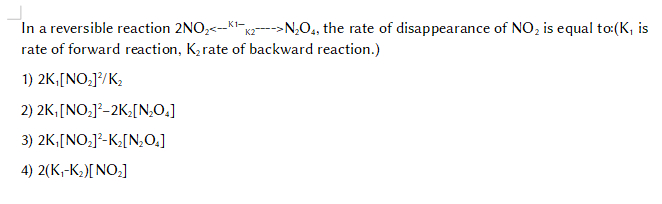
Asked by koushikr063 | 06 May, 2019, 10:37: AM
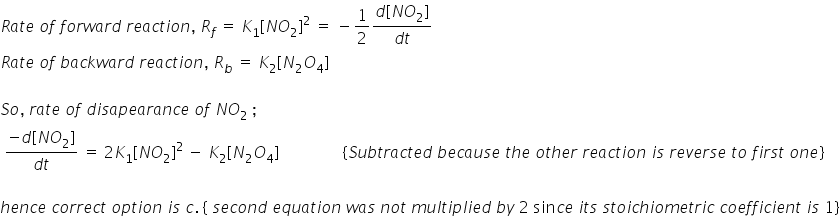
Answered by Sumit Chakrapani | 06 May, 2019, 11:21: AM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by neerajavuppala1983 | 23 Jul, 2024, 22:49: PM
JEE main - Chemistry
Asked by tanniruv133 | 03 Jul, 2024, 18:50: PM
JEE main - Chemistry
Asked by heyyyyy | 12 Jun, 2024, 19:18: PM
JEE main - Chemistry
Asked by hv5594265 | 12 Jun, 2024, 11:59: AM
JEE main - Chemistry
Asked by rupalibhange1987 | 11 Jun, 2024, 20:00: PM
JEE main - Chemistry
Asked by gajju8493 | 11 Jun, 2024, 15:09: PM
JEE main - Chemistry
Asked by chakrabortymithu041 | 29 May, 2024, 18:45: PM













