JEE Class main Answered
If the given reaction is carried out with 1 mol N2 and excess of H2 at a constant pressure of 40 bar then volume contraction of 2 L is observed. [Given : N2 (g) + 3H2 (g) ? 2NH3 ?H° = – 90 kJ] Now if 2 mol N2 and 8 mol H2 are taken then calculate (a) PV work done (b) ?U° for given amount
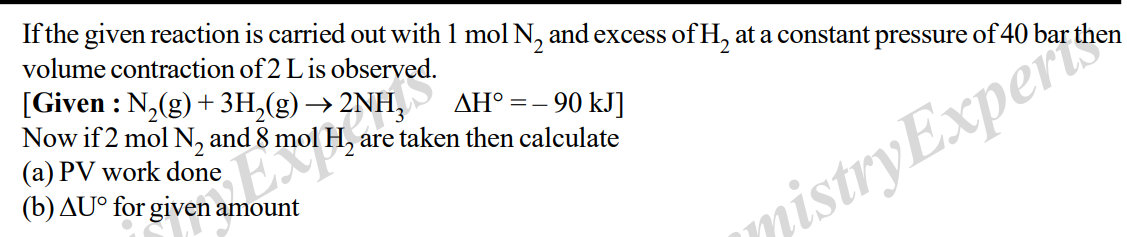
Asked by kartikey.zopcart | 30 Oct, 2021, 16:17: PM
Based on the values of B.E. given, ΔfH0 of N2H4(g) is :
Given BE of :
N−N is 159 kJ mol−1,
H-H is 436 kJ mol−1,
N≡N is 941 kJ mol−1,
N−H is 398 kJ mol−1
N2 (g) + 2H2 (g) → 2N2H2
ΔfH0 of N2H4(g) = 941 + (2× 436) - (4×398 +159)
= 1813 - 1751
= 62 kg mol-1
Answered by Ramandeep | 30 Oct, 2021, 19:07: PM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by neerajavuppala1983 | 23 Jul, 2024, 22:49: PM
JEE main - Chemistry
Asked by tanniruv133 | 03 Jul, 2024, 18:50: PM
JEE main - Chemistry
Asked by heyyyyy | 12 Jun, 2024, 19:18: PM
JEE main - Chemistry
Asked by hv5594265 | 12 Jun, 2024, 11:59: AM
JEE main - Chemistry
Asked by rupalibhange1987 | 11 Jun, 2024, 20:00: PM
JEE main - Chemistry
Asked by gajju8493 | 11 Jun, 2024, 15:09: PM
JEE main - Chemistry
Asked by chakrabortymithu041 | 29 May, 2024, 18:45: PM













