CBSE Class 12-science Answered
Hybridization of Xe & Sb in the product, when XeF4 react with SbF5 respectively
Asked by Atulcaald | 18 May, 2018, 01:23: AM
XeF4 reacts with SbF5 which acts as a Lewis acid and gives,
XeF4 + SbF5 → [XeF3]+ [SbF6]-
The hybridization of [XeF3]+=Trigonal bipyramidal = sp3d2
The hybridization of [SbF6]- = Octahedral =sp3d2
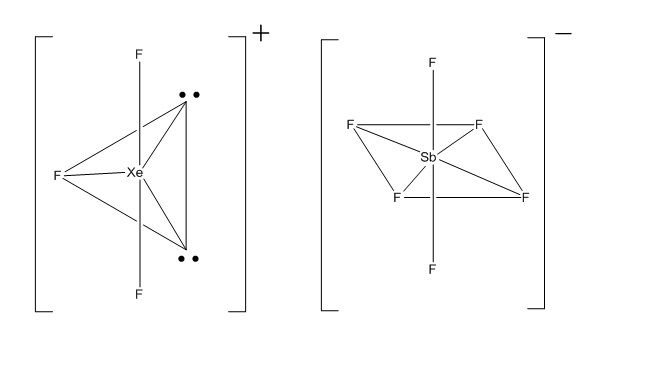
Answered by Ramandeep | 18 May, 2018, 02:38: PM
CBSE 12-science - Chemistry
Asked by parveendsw | 28 Sep, 2019, 08:26: AM
CBSE 12-science - Chemistry
Asked by tweetydash01 | 12 Aug, 2018, 06:11: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 18 May, 2018, 01:23: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 09:51: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 12 Jun, 2014, 01:13: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 12 Jun, 2014, 01:14: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 12 Jun, 2014, 01:16: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 09:52: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 12 Jun, 2014, 01:20: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 12 Jun, 2014, 01:21: PM

