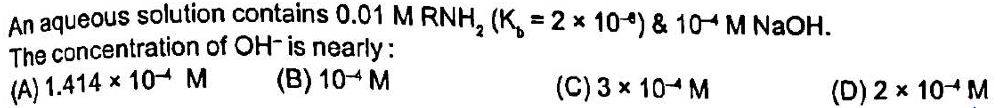JEE Class main Answered
Explanation and answer of this question?
Asked by Yasharthshankar121 | 29 Jun, 2020, 18:31: PM
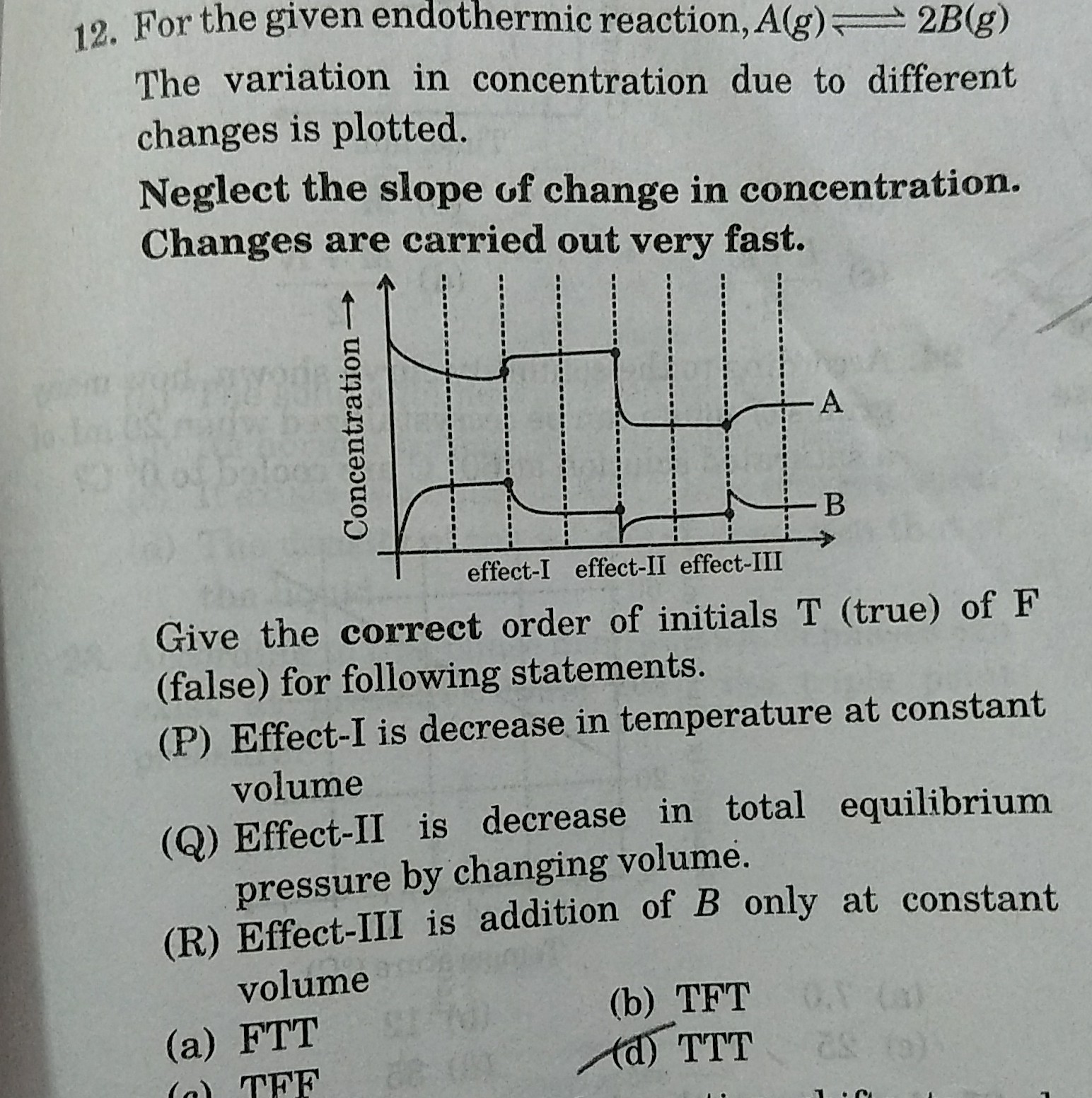
Effect I-There will be decrease in temperature at constant volume. The reaction will proceed in the backward direction, the reactant concentration will increase and the product concentration will decrease.
Effect II -It corresponds to the addition of inert gas at constant pressure. The equilibrium will remain unaffected and the concentrations of A and B will not be affected by this change.
Effect III- It corresponds to a decrease in total equilibrium pressure by changing the volume. The equilibrium will shift in the forward direction so that the pressure increases.
Effect IV-It corresponds to the addition of B only at constant volume. The reaction will proceed in the backward direction, the reactant concentration will increase and the product concentration will decrease.
Effect II -It corresponds to the addition of inert gas at constant pressure. The equilibrium will remain unaffected and the concentrations of A and B will not be affected by this change.
Effect III- It corresponds to a decrease in total equilibrium pressure by changing the volume. The equilibrium will shift in the forward direction so that the pressure increases.
Effect IV-It corresponds to the addition of B only at constant volume. The reaction will proceed in the backward direction, the reactant concentration will increase and the product concentration will decrease.
Therefore, the correct option is D
Answered by Ravi | 30 Jun, 2020, 10:52: AM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by mokayesu162 | 26 Nov, 2023, 13:40: PM
JEE main - Chemistry
Asked by kumardevanand11825 | 04 Jun, 2022, 14:07: PM
JEE main - Chemistry
Asked by pritgambhwa | 31 Dec, 2021, 16:36: PM
JEE main - Chemistry
Asked by gargchahat2005 | 13 Dec, 2020, 17:16: PM
JEE main - Chemistry
Asked by Yasharthshankar121 | 29 Jun, 2020, 18:31: PM
JEE main - Chemistry
Asked by rgunasekhar2222 | 26 Jun, 2020, 08:46: AM
JEE main - Chemistry
Asked by monjittaye16 | 23 Apr, 2020, 17:10: PM