JEE Class main Answered
At temp T a co
mpound AB2(g) dissociates according to the reaction
2AB2→2AB+B2 with a degree of dissociation X which is small as compared to unity .the expression for Kpin terms of X and total pressure P is
Asked by ak020820002 | 05 Feb, 2019, 00:48: AM
The compound AB2(g) dissociates according to the reaction,
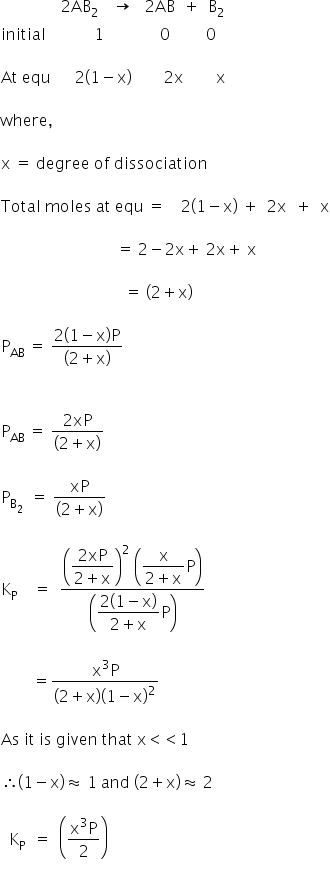
The equilibrium constant Kp = (x3P)/2
Answered by Varsha | 05 Feb, 2019, 11:25: AM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by neerajavuppala1983 | 23 Jul, 2024, 22:49: PM
JEE main - Chemistry
Asked by tanniruv133 | 03 Jul, 2024, 18:50: PM
JEE main - Chemistry
Asked by heyyyyy | 12 Jun, 2024, 19:18: PM
JEE main - Chemistry
Asked by hv5594265 | 12 Jun, 2024, 11:59: AM
JEE main - Chemistry
Asked by rupalibhange1987 | 11 Jun, 2024, 20:00: PM
JEE main - Chemistry
Asked by gajju8493 | 11 Jun, 2024, 15:09: PM
JEE main - Chemistry
Asked by chakrabortymithu041 | 29 May, 2024, 18:45: PM













