JEE Class main Answered
Ag is movalent with atomic mass 108 Cu is divalent with atomic mass 63.6. The same electric current is passed for the same length of time through a Ag coulometer and a Cu coulometer which are connected in series. If 27g of Ag is deposited, then the corresponding amount of Cu deposited is
A.
63.6g
B.
31.8g
C.
15.9g
D.
7.95g
Asked by s.ojaswini17 | 14 Feb, 2019, 20:44: PM
According to faradays law, the mass of metal depposited is directly proportional to its equivalent mass so, we can write it as -
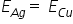
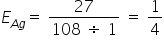 { 1 Being the Valency Factor For Ag}
{ 1 Being the Valency Factor For Ag}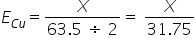 (2 Being the valency Factor for Cu} And [Eqivalent = No of moles ÷ Valency factor]
(2 Being the valency Factor for Cu} And [Eqivalent = No of moles ÷ Valency factor]So, Comparing the above two we get X = 7.9375g. (option d).
Answered by Sumit Chakrapani | 15 Feb, 2019, 02:47: AM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by neerajavuppala1983 | 23 Jul, 2024, 22:49: PM
JEE main - Chemistry
Asked by tanniruv133 | 03 Jul, 2024, 18:50: PM
JEE main - Chemistry
Asked by heyyyyy | 12 Jun, 2024, 19:18: PM
JEE main - Chemistry
Asked by hv5594265 | 12 Jun, 2024, 11:59: AM
JEE main - Chemistry
Asked by rupalibhange1987 | 11 Jun, 2024, 20:00: PM
JEE main - Chemistry
Asked by gajju8493 | 11 Jun, 2024, 15:09: PM
JEE main - Chemistry
Asked by chakrabortymithu041 | 29 May, 2024, 18:45: PM













