NEET Class neet Answered
A graph is plotted by taking pressure along y-axis and centigrade temperature along x-axis for an ideal gas at constant volume. x intercept of the graph is
Asked by harinderamit1234 | 01 Feb, 2020, 09:35: PM
ideal gas equation :- PV = nRT
where P is pressure , V is volume, n is number of moles, R is gas constant, and T is temperature in kelvin
at constant volume, P = (nR/V) T or P = c T where c is constant
Plot of P Vs T ( when P is taken in Y-axis) gives x-intercet as 0 K ( -273 o C ), i.e. value of T when P=0 .
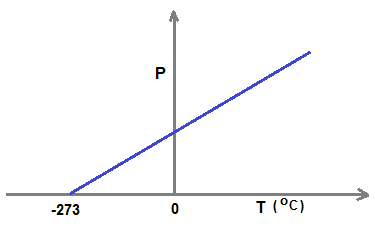
Answered by Thiyagarajan K | 02 Feb, 2020, 08:34: AM
Application Videos
NEET neet - Physics
Asked by bidyutpravarout79 | 26 Apr, 2024, 09:40: PM
NEET neet - Physics
Asked by ramanjaneyuluoguru | 25 Apr, 2024, 04:18: PM
NEET neet - Physics
Asked by shatakshibhatt9 | 20 Apr, 2024, 07:52: PM
NEET neet - Physics
Asked by praveenpriya000079 | 18 Apr, 2024, 07:24: AM
NEET neet - Physics
Asked by gouranshi84 | 17 Apr, 2024, 05:23: PM
NEET neet - Physics
Asked by sojusvi | 17 Apr, 2024, 01:12: PM