ICSE Class 7 Answered
561dm3 of a gas at STP is filked una 748dm3 container.If temperature is constant, Calculate the percentage change in pressure required.
Asked by Jeevan | 06 Jan, 2018, 09:20: PM
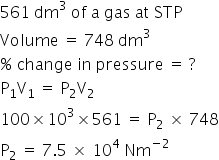
Answered by Ramandeep | 07 Jan, 2018, 02:13: PM
Concept Videos
ICSE 7 - Chemistry
Asked by dhakankar | 25 Nov, 2023, 08:08: PM
ICSE 7 - Chemistry
Asked by nitinshetes | 10 Feb, 2021, 12:00: PM
ICSE 7 - Chemistry
Asked by poojaaggarwalmail | 22 Aug, 2020, 07:45: AM
ICSE 7 - Chemistry
Asked by Shaikfarzana6228 | 20 Feb, 2020, 06:50: AM
ICSE 7 - Chemistry
Asked by Molaypaul700 | 12 Nov, 2019, 06:34: PM
ICSE 7 - Chemistry
Asked by nagmajahangir786 | 06 Nov, 2019, 08:22: PM
ICSE 7 - Chemistry
Asked by jahangirhossain848 | 17 Oct, 2019, 03:56: PM
ICSE 7 - Chemistry
Asked by jahangirhossain848 | 09 Oct, 2019, 06:17: PM
ICSE 7 - Chemistry
Asked by sabinapriyesh4 | 23 Sep, 2018, 07:39: AM
ICSE 7 - Chemistry
Asked by Jeevan | 06 Jan, 2018, 09:20: PM