JEE Class main Answered
10 moles of purePCIs gas is put into a closed container of volume V' and temperature 'T' and allowed to come to equilibrium, at an equilibrium pressure of 20 atm. The pure PCls is found to be 50% dissociated at equilibrium. PCls(g) = PCl3(g) + Cl2(9) find kp
Asked by ironmangroot3000 | 05 Dec, 2021, 11:37: AM
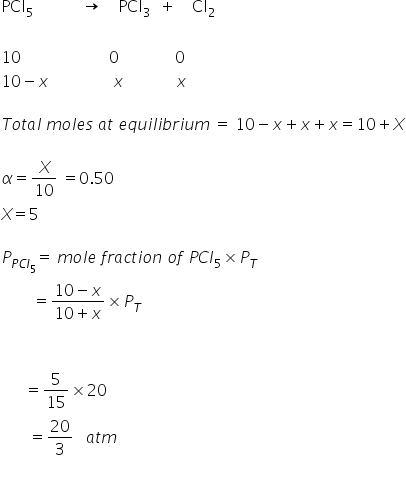
Answered by Ravi | 12 Dec, 2021, 21:16: PM
JEE main - Chemistry
Asked by ironmangroot3000 | 05 Dec, 2021, 11:37: AM
