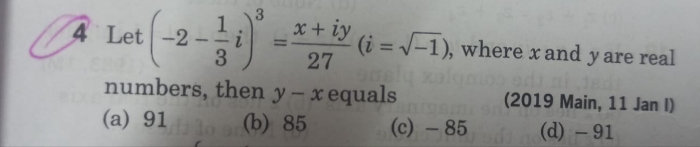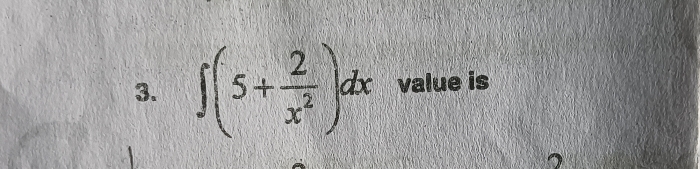Doubts and Solutions
OR
CBSE XI Commerce - Business Studies
Asked by papunpattanayak143 | 04 May, 2024, 01:46: AM
JEE Main - Maths
Asked by aniyoggavhane38 | 03 May, 2024, 09:41: PM
JEE Main - Maths
Asked by priyadharshinigopinath27 | 03 May, 2024, 08:44: PM
CBSE XI Science - Maths
Asked by medanagaraju2022 | 03 May, 2024, 07:37: PM
CBSE VII - Science
Asked by nishakmishra | 03 May, 2024, 05:43: PM
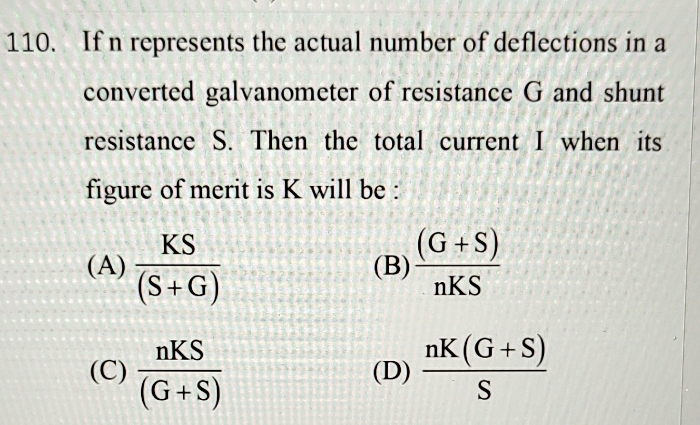
JEE Main - Physics
Asked by sandhyapallapu22 | 03 May, 2024, 04:32: PM
NEET NEET - Physics
Asked by hardikmittal25 | 03 May, 2024, 02:57: PM
JEE Main - Maths
Asked by navyagoel052 | 03 May, 2024, 02:51: PM