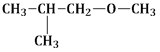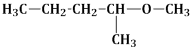Question Paper (Section wise)
-
1) The mean free path of molecules of a gas, (radius ‘r’) is inversely proportional to:-
-
r3
-
r2
-
R
-
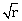
-
-
2) If n1, n2, and n3 are the fundamental frequencies of three segments into which a string is divided, then the original fundamental frequency n of the string is given by –
-
-
3) The number of possible natural oscillations of air column in a pipe closed at one end of length 85 cm whose frequencies lie below 1250 Hz are: (Velocity of sound = 340 ms-1)
-
4
-
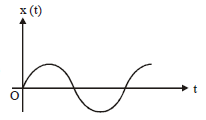
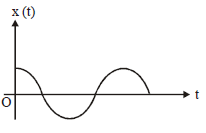
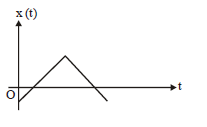
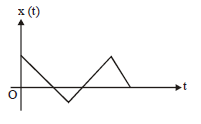
5
-
7
-
6
-
-
4) A speeding motorcyclist sees traffic jam ahead of him. He slows down to 36 km/hour. He finds that traffic has eased and a car moving ahead of him at 18 km/hour is honking at a frequency of 1392 Hz. If the speeds of sound is 343 m/s, the frequency of the honk as heard by him will be
-
1332 Hz
-
1372 Hz
-
1412 Hz
-
1454 Hz
-
-
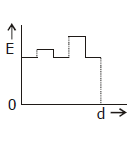
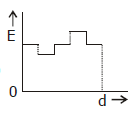
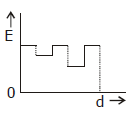
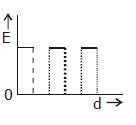
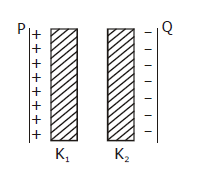
5) Two thin dielectric slabs of dielectric constants K2 and K2 (K1 < K2) are interested between plates of a parallel plate capacitor, as shown in the figure. The variation of electric field ‘E’ between the plates with distance ‘d’ as measured from plate P is correctly shown by:
-
-
6) A conducting sphere of radius R is given a charge Q. The electric potential and the electric field at the center of the sphere respectively are –
-
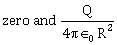
-
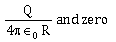
-
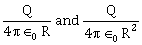
-
Both and zero
-
-
7) In a region, the potential is represented by V(x, y, z) = 6x - 8xy – 8y + 6yz, where V is in volts and x, y, z are in meters. The electric force experienced by a charge of 2 coulomb situated at point (1, 1, 1) is –
-
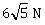
-
30 N
-
24 N
-
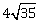
-
-
8) Two cities are 150 km apart. Electric power is sent from one city to another city through copper wires. The fall of potential per km is 8 volt and the average resistance per km is 0.5 Ω. The power loss in the wires is-
-
19.2 W
-
19.2 kW
-
19.2 J
-
12.2 kW
-
-
9) The resistance in the two arms of the meter bridge are 5Ω and RΩ, respectively. When the resistance R is shunted with an equal resistance, the new balance point is at 1.6 ℓ1. The resistance ‘R’ is-
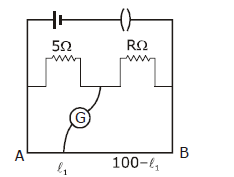
-
100Ω
-
15Ω
-
20Ω
-
25Ω
-
-
10) A bar magnet of length ‘ℓ’ and magnetic dipole moment ‘M’ is bent in the form of an arc as shown in figure. The new magnetic dipole moment will be-
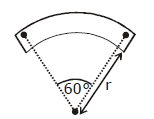
-
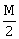
-
M
-
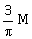
-
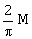
-
-
11) The internal resistance of a 2.1 V cell which gives a current of 0.2 V cell which gives a current of 0.2 A through a resistance of 10Ω is-
-
1.0 Ω
-
0.2 Ω
-
0.5 Ω
-
0.8 Ω
-
-
12) For photoelectric emission from certain metal the cutoff frequency is v. If radiation of frequency 2v impinges on the metal plate, the maximum possible velocity of the emitted electron will be (m is the electron mass)-
-
-
13) During an adiabatic process, the pressure of a gas found to be proportional to the cube of its temperature. The ratio of
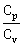 for the gas is –
for the gas is –-
3/2
-
4/3
-
2
-
5/3
-
-
14) The following four wires are made of the same material. Which of these will have the largest extension when the same tension is applied?
-
length = 300 cm, diameter = 3 mm
-
length = 50 cm, diameter = 0.5 mm
-
length = 100cm, diameter = 1 mm
-
length = 200 cm, diameter = 2 mm
-
-
15) The resistances of the four arms P,Q,R and S in a Wheatstone’s bridge are 10 ohm, 30 ohm, 30 ohm and 90 ohm, respectively. The e. m. f. and internal resistance of the cell are 7 volt and 5 ohm respectively. If the galvanometer resistance is 50 ohm, the current drawn from the cell will be-
-
2.0 A
-
1.0 A
-
0.2 A
-
0.1 A
-
-
16) The amount of heat energy required to raise the temperature of 1 g of Helium at NTP, from T1 K to T2 K is-
-
-
17) A piece of iron is heated in a flame. It first become dull red then becomes reddish yellow and finally turns to white hot. The correct explanation for the above observation is possible by using:-
-
Newton’s law of cooling
-
Stefan’s law
-
Wien’s law
-
Kirchhoff’s law
-
-
18) A gas taken through the cycle A → B → C → A, as shown, what is the net work done by the gas?
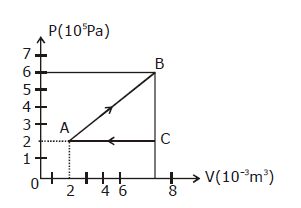
-
-2000 J
-
2000 J
-
1000 J
-
zero
-
-
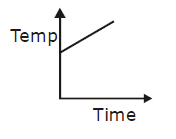
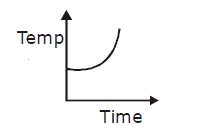
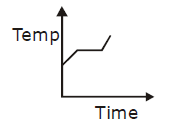
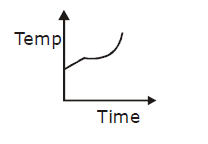
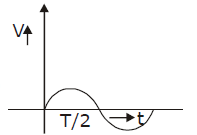
19) Liquid oxygen at 50 K is heated to 300 K at constant pressure of 1 atm. The rate of heating is constant. Which one of the following graphs represents the variation of temperature with time?
-
-
20) If the radius of a star is r and it acts as a black body, what would be the temperature of the star, in which the rate of energy production is Q?
(σ stands for Stefan’s constant.)
-
(4πR2Q / σ)1/4
-
(Q / 4πR2σ)1/4
-
Q / 4πR2σ
-
(Q / 4πR2σ)-1/2
-
-
21) A coil of resistance 400Ω is placed in a magnetic field. If the magnetic flux ϕ (Wb) linked with the coil varies with time t (sec) as
Ф = 50t2 + 4
The current in the coil at t = 2 s is:
-
2A
-
1A
-
0.5A
-
0.1A
-
-
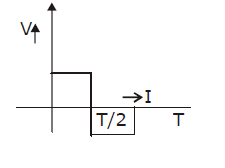
22) The current (I) in the inductance is varying with time according to the plot shown in figure.
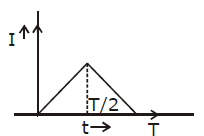
Which one of the following is the correct variation of voltage with time in the coil?
-
-
23) In a electrical circuit R, L, C and a.c voltage source are all connected in series. When L is removed from the circuit, the phase difference between the voltage and the current in the circuit is π/3. If instead, C is removed from the circuit the phase difference is again π/3. The power factor of the circuit is:
-
1
-
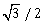
-
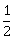
-
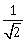
-
-
24) A ring is made of a wire having a resistance Ro = 12Ω. Find the points A and B as shown in the figure at which a current carrying conductor should be connected so that the resistance R of the sub circuit between these point is equal to 8/3 Ω.
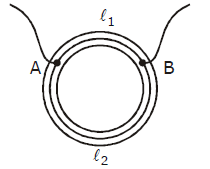
-
-
25) If voltage across a bulb rated 220 Volt 100 what drops by 2.8 % of its rated value, the percentage of the rated value by which the power would decrease is
-
5%
-
10%
-
20%
-
2.5%
-
-
26) In the circuit shown the cells A and B have negligible resistances. For VA = 12V, R1 = 500 Ω and R = 100 Ω the galvanometer (G) shows to deflection. The value of VB is
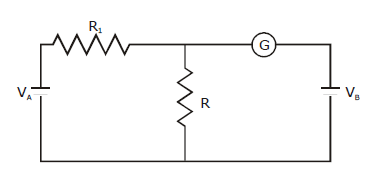
-
12V
-
6V
-
4V
-
2V
-
-
27) The electric field associated with an EM wave in vacuum is given by
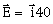 cos (kz – 6 × 108t), where E < z and t are in volt/m, meter and seconds respectively. The value of wave vector k is:
cos (kz – 6 × 108t), where E < z and t are in volt/m, meter and seconds respectively. The value of wave vector k is:-
6m-1
-
3 m-1
-
2 m-1
-
0.5 m-1
-
-
28) The ratio of amplitude magnetic field to the amplitude of electric field for an electromagnetic wave propagating in vacuum is equal to –
-
The ratio of magnetic permeability to the electric susceptibility of vacuum
-
Unity
-
The speed of light in vacuum
-
Reciprocal of speed of light in vacuum
-
-
29) A car of mass m starts from rest and accelerates so that the instantaneous power delivered to the car has a constant magnitude P0. The instantaneous velocity of this car is proportional to –
-
t-1/2
-
-
t2P0
-
t1/2
-
-
30) The moment of inertia of a uniform circular disc is maximum about an axis perpendicular to the disc and passing through –
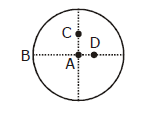
-
D
-
A
-
B
-
C
-
-
31) Three masses are placed on the x-axis: 300 mg at origin, 500 mg at x = 40 cm and 400 mg at x = 70 cm. The distance of the center of mass from the origin is –
-
50 cm
-
30 cm
-
40 cm
-
45 cm
-
-
32) A car of mass m is moving on a level circular track of radius R. If µs represents the static friction between the road and tyres of the car, the maximum speed of the car in circular motion is given by –
-
-
33) An ideal gas goes from state A to state B via three different processes as indicated in the P-V diagram. If Q1,Q2,Q3 indicated the heat absorbed by the gas along the three processes and ∆U1, ∆U2, ∆U3 three processes respectively, then –
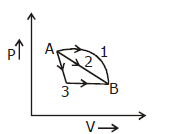
-
Q1 = Q2 = Q3 and ∆U1 > ∆U2 > ∆U3
-
Q1 > Q2 > Q3 and ∆U1 = ∆U2 = ∆U3
-
Q1 > Q2 > Q3 and ∆U1 = ∆U2 = ∆U3
-
Q1 = Q2 = Q3 and ∆U1 > ∆U2 > ∆U3
-
-
34) A stone is dropped from a height h. It hits the ground with a certain momentum P. If the same stone is dropped from a height 100 % more than the previous height, the momentum when it hits the ground will changed by-
-
200 %
-
100 %
-
68 %
-
41 %
-
-
35) The instantaneous values of altering current and voltage in a circuit are given as
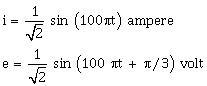
The average power in watts consumed in the circuit is -
-
-
36) If the momentum of an electron is changed by P, then the de-Broglie wavelength associated with it changed by 0.5%. The initial momentum of electron will be-
-
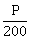
-
100 P
-
200 P
-
400 P
-
-
37) When 1 kg of ice at 0°C melts to water at 0°C, the resulting change in its entropy, taking latent heat of ice to be 80 cal/°C is-
-
293 cal/K
-
273 cal/K
-
8 × 104 cal/K
-
80 cal/K
-
-
38) During an isothermal expansion, a confined ideal gas does –150 J of work against its surrounding. This implies that
-
150 J of heat has been added to the gas
-
150 J of heat has been removed from the gas
-
300 J of heat has been added to the gas
-
No heat is transferred because the process is isothermal
-
-
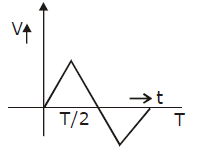
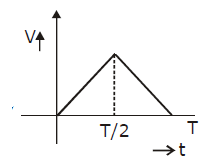
39) A particle of mass m is released from rest and follows a parabolic path as shown. Assuming that the displacement of the mass from the origin is small, which graph correctly depicts the position of the particle as a function of time?
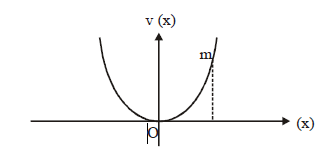
-
-
40) Two waves are represented by the equations y1= Asin (ωt + kx + 0.57) m and y2 = Acos (ωt + kx) m, is difference between them is-
-
0.57 radian
-
1.0 radian
-
1.25 radian
-
1.57 radian
-
-
41) Out of the following functions representing motion of a particle which represents SHM?
(A) y = sin ωt – cosωt
(B) y = sin3ωt
(C)
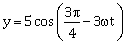
(D) y = 1 + ωt + ω2t2
-
Only (A) and (B)
-
Only (A)
-
Only (D) does not represent SHM
-
Only (A) and (C)
-
-
42) Sound waves travel at 350 m/s through a warm air and at 3500 m/s through brass. The wavelength of a 700 Hz acoustic wave as it enters brass from warm air
-
Decrease by a factor 20
-
Decreases by a factor 10
-
Increases by a factor 20
-
Increases by a factor 10
-
-
43) The decreasing order of infrared, microwave, ultraviolet and gamma rays is-
-
Infrared, microwave, ultraviolet, gamma rays
-
Microwave, infrared, ultraviolet, gamma rays
-
Gamma rays, ultraviolet, infrared, microwaves
-
Microwaves, gamma rays, infrared, ultraviolet
-
-
44) The wavelenght of the first line of Lyman series for hydrogen atom is equal to that of the second line of Balmer series for a hydrogen like ion. The atomic number Z of hydrogen like ion is
-
2
-
3
-
4
-
1
-
-
45) Which of the following is not due to total internal reflection?
-
Brilliance of diamond
-
Working of optical fiber
-
Difference between apparent and real depth of pond
-
Mirage on hot summer days
-
-
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
-
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
-
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
-
1) Be2+ is isoelectronic with which of the following ions?
-
H+
-
Li+
-
Na+
-
Mg2+
-
-
2) Which of the following molecules has the maximum dipole moment?
-
CO2
-
CH4
-
NH3
-
NF3
-
-
3) Which one of the following species has plane triangular shape?
-
N3
-
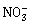
-
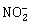
-
CO2
-
-
4) Acidity of diprotic acids in aqueous solutions increases in the order:–
-
H2S < H2Se < H2Te
-
H2Se < H2S < H2Te
-
H2Te < H2S < H2Se
-
H2Se < H2Te < H2S
-
-
5) (a) H2O2 + O3 → H2O + 2O2
(b) H2O2 + Ag2O → 2Ag + H2O + O2
Role of hydrogen peroxide in the above reactions is respectively-
-
Oxidizing in (a) and reducing in (b)
-
Reducing in (a) and oxidizing in (b)
-
Reducing in (a) and (b)
-
Oxidizing in (a) and (b)
-
-
6) Artificial sweetener which is stable under cold conditions only is:–
-
Saccharine
-
Sucralose
-
Aspartame
-
Alitame
-
-
7) In acidic medium, H2O2 changes Cr2O7–2 to CrO5 which has two (–O–O) bonds. Oxidation state of Cr in CrO5 is:–
-
+5
-
+3
-
+6
-
-10
-
-
8) The reaction of aqueous KMnO4 with H2O2 in acidic conditions gives:–
-
Mn4+ and O2
-
Mn2+ and O2
-
Mn2+ and O3
-
Mn4+ and MnO2
-
-
9) Among the following complexes the one which shows zero crystal field stabilization energy (CFSE) is:–
-
[Mn (H2O)6]3+
-
[Fe (H2O)6]3+
-
[Co (H2O)6]2+
-
[Co (H2O)6]3+
-
-
10) Which of these is not a monomer for a high molecular mass silicone polymer?
-
PhSiCl3
-
Me2 SiCl2
-
MeSiCl3
-
Me3SiCl
-
-
11) A reaction having equal energies of activation for forward and reverse reactions has:-
-
∆H = ∆G = ∆S = 0
-
∆S = a
-
∆G = 0
-
∆H = 0
-
-
12) At 25°C molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 ohm-1 cm2 mol-1 and at infinite dilution its molar conductance is 238 ohm-1 cm2 mol-1. The degree of ionization of ammonium hydroxide at the same concentration and temperature is:-
-
40.800%
-
2.080%
-
20.800%
-
4.008%
-
-
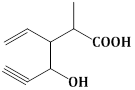
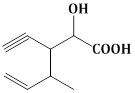
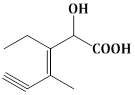
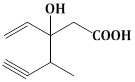
13) Structure of the compound whose IUPAC name is 3-Ethyl-2-hydroxy-4-methylhex- 3- en-5-ynoic acid is:-
-
-
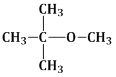
14) Among the following ethers, which one will produce methyl alcohol on treatment with hot concentrated Hl?
-
-
15) Antiseptics and disinfectants either kill or prevent growth of microganisms. Identify which of the following statements is not true:-
-
Disinfectants harm the living tissues
-
A 0.2 % solution of phenol is an antiseptic while 1% solution acts as a disinfectant
-
Chlorine and Iodine are used as strong disinfectants
-
Dilute solutions of Boric acid and Hydrogen Peroxide are strong antiseptics
-
-
16) A magnetic moment of 1.73 BM will be shown by one among the following:-
-
[CoCl6]4-
-
[Cu(NH3)4]2+
-
[Ni(CN)4]2-
-
TiCI4
-
-
17) KMnO4 can be prepared from K2MnO4 as per the reaction:-
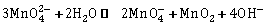
The reaction can go to completion by removing OH− ions by adding:
-
SO2
-
HCI
-
KOH
-
CO2
-
-
18) Reaction by which Benzaldehyde cannot be prepared:-
-
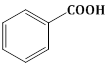 + Zn/Hg and conc. HCI
+ Zn/Hg and conc. HCI -
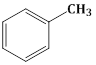 + CrO2 CI2 in CS2 followed by H3O⊕
+ CrO2 CI2 in CS2 followed by H3O⊕ -
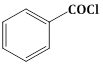 + H2 in presence of Pd + BaSO4
+ H2 in presence of Pd + BaSO4 -
 + CO + HCI in presence of anhydrous AICI3
+ CO + HCI in presence of anhydrous AICI3
-
-
19) A metal crystallizes with a face- centered cubic lattice. The edge of the unit cell is 408pm. The diameter of the metal atom is:
-
144 pm
-
204 pm
-
288 pm
-
408 pm
-
-
20) The enthalpy of fusion of water is 1.435 kcal/mol. The molar entropy change for the melting of ice at 0° C is:
-
5.260 cal/(mol K)
-
0.526 cal/(mol K)
-
10.52 cal/(mol K)
-
21.04 cal/(mol K)
-
-
21) In which of the following compounds, nitrogen exhibits highest oxidation state?
-
N3H
-
NH2OH
-
N2H4
-
NH3
-
-
22) Aluminium is extracted from alumina (Al2O3) by electrolysis of a molten mixture of:
-
Al2O3 +Na3AlF6 + CaF2
-
Al2O3 + KF +Na3AlF6
-
Al2O3 + HF + NaAlF4
-
Al2O3 + CaF2 + NaAlF4
-
-
23) Which of the statements is not true?
-
K2Cr2O7 solution in acidic medium is orange
-
K2Cr2O7 solution becomes yellow on increasing the pH beyond 7
-
On passing H2S through acidified K2Cr2O7 solution, a milky colour is observed
-
Na2Cr2O7 is preferred over K2Cr2O7 in volumetric analysis
-
-
24) A mixture of potassium chlorate, oxalic acid and sulphuric acid is heated. During the reaction which element undergoes maximum change in the oxidation number?
-
Cl
-
C
-
S
-
H
-
-
25) Which one of the following is an outer orbitals complex and exhibits paramagnetic behavior?
-
[Cr(NH3)6]3+
-
[CO(NH3)6]3+
-
[Ni(NH3)6]2+
-
[Zn(NH3)6]2+
-
-
26) The ease of adsorption of the hydrated alkali metal ions on an ion-exchange resins follows the order:
-
K+ < Na+ < Rb+ < Li+
-
Na+ < Li+ < K+ < Rb+
-
Li+ < K+ < Na+ < Rb+
-
Rb+ < K+ < Na+ < Li+
-
-
27) Equimolar solutions of the following substances were prepared separately. Which one of these will record the highest pH value?
-
LiCl
-
BeCl2
-
BaCl2
-
AlCl3
-
-
28) Which one of the following sets forms the biodegradable polymer?
-
HO – CH2 – CH2 – OH and
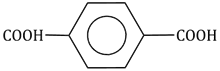
-
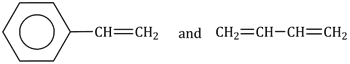
-
CH2 = CH – CN and CH2 = CH – CH = CH2
-
H2N – CH2 – COOH and H2N – (CH2)5 -COOH
-
-
29) Consider the reaction
RCHO + NH2NH2 → RCH = N – NH2
What sort of reaction is it?
-
Electrophilic substitution- elimination reaction
-
Nucleophilic addition- elimination reaction
-
Electrophilic addition- elimination reaction
-
Free radical addition - elimination reaction
-
-
30) Consider the following reaction:
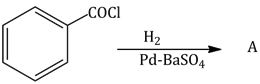
The product ‘A’ is:
-
C6H5COCH3
-
C6H5Cl
-
C6H5CHO
-
C6H5OH
-
-
31) Standard reduction potentials of the half reactions are given below:
F2(g) + 2e- → 2F- (aq) ; E° = + 2.85 V
Cl2(g) + 2e- → 2Cl- (aq) ; E° = + 1.36 V
Br2(ℓ) + 2e- → 2Br- (aq) ; E° = + 1.06 V
I2(s) + 2e- → 2I- (aq) ; E° = + 0.53 V
The strongest oxidizing and reducing agents respectively are:
-
Cl2 and Br-
-
Cl2 and I2
-
F2 and I-
-
Br2 and CI-
-
-
32) Structure of a mixed oxide is cubic close packed (c. c. p.). The cubic unit cell of mixed oxide is composed of oxide ions. One fourth of the tetrahedral voids are occupied by divalent metal A and the octahedral voids are occupied by a monovalent metal B. The formula of the oxide is:
-
A2B3O4
-
AB2O2
-
ABO2
-
A2BO2
-
-
33) Activation energy (Ea) and rate constant (k1 and k2) of a chemical reaction at two different temperatures (T1 and T2) are related by:
-
-
34) Equal volumes of two monoatomic gases, A and B, at same temperature and pressure are mixed. The ratio of specific heats (Cp/Cv) of the mixture will be:-
-
3. 3
-
1. 67
-
0. 83
-
1. 50
-
-
35) In which of the following arrangements the given sequence is not strictly according to the property indicated against it?
-
NH3 < PH3 < AsH2 < AbH3:
Increasing acidic character
-
CO2 < SiO2 < SnO2 < PbO2
Increasing oxidizing power
-
HF < HCl < HBr < HI:
Increasing acidic strength
-
H2O < H2S < H2Se < H2Te
Increasing pKa values
-
-
36) Molar conductivities (∧ °m) at infinite dilution of NaCl, HCl and CH3COONa are 126.4, 425.9 and 91.0 s cm2 mol-1 respectively. ∧ °m for CH3COOH will be:-
-
290.8 s cm2mol-1
-
390.5 s cm2mol-1
-
425.5 s cm2mol-1
-
180.5 s cm2mol-1
-
-
37) Which one of the following is present as an active ingredient in bleaching powder for bleaching action?
-
CaCl2
-
CaOCl2
-
Ca(OCl)2
-
CaO2Cl
-
-
38) The complex, [Pt(Py)(NH3)BrCl] will have how many geometrical isomers?
-
2
-
3
-
4
-
0
-
-
39) Name the type of the structure of silicate in which one oxygen atom of [SiO4]4- is shared?
-
Three dimensional
-
Linear chain silicate
-
Sheet silicate
-
Pyrosilicate
-
-
40) The complex [Co(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [Co(CN)6] are the examples of which type of isomerism?
-
Geometrical isomerism
-
Linkage isomerism
-
Ionization isomerism
-
Coordination isomerism
-
-
41) The d-electron configurations of Cr2+, Mn2+, Fe2+ and Co2+ are d4, d5, d6 and d7 respectively. Which one of the following will exhibit minimum paramagnetic behavior?
-
[Cr (H2O)6]3+
-
[Mn (H2O)6]2+
-
[Fe (H2O)6]2+
-
[Co (H2O)6]2+
-
-
42) Of the following complex ions, which is diamagnetic in nature?
-
[CoF6]3-
-
[NiCl4]2-
-
[Ni (CN)4]2-
-
[CuCl4]2-
-
-
43) Which of the following has the minimum bond length?
-
O2
-
O2+
-
O2–
-
O22-
-
-
44) The value of ∆H for the reaction
X2 (g) + 4Y2 (g) ⇌ 2XY4 (g) is less than zero.
Formation of XY4 (g) will be favoured at
-
High pressure and low temperature
-
High temperature and high pressure
-
Low pressure and low temperature
-
High temperature and low pressure
-
-
45) Of the following which one is classified as polyester polymer?
-
Nylon – 66
-
Terylene
-
Backelite
-
Melamine
-
-
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
-
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
-
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
-
1) Assisted reproductive technology, IVF involves transfer of:
-
Zygote into the fallopian tube.
-
Zygote into the uterus
-
Embryo with 16 blastomeres into the fallopian tube
-
Ovum into the fallopian tube
-
-
2) An example of ex situ conservation is:
-
Seed bank
-
Wildlife sanctuary
-
Sacred grove
-
National park
-
-
3) The osmosis of a cell kept in water is chiefly regulated by:
-
Vacuoles
-
Plastids
-
Ribosomes
-
Mitochondria
-
-
4) Which one of the following is wrong about Chara?
-
Globule and nucule present on the same plant.
-
Upper antheridium and lower oogonium.
-
Globule is the male reproductive structure.
-
Upper oogonium and lower round antheridium.
-
-
5) The first human hormone produced by recombinant DNA technology is:
-
Estrogen
-
Thyroxine
-
Progesterone
-
Insulin
-
-
6) Which one of the following statements is not correct?
-
In retina the rods have the photo pigment rhodopsin while cones have three different photo pigments.
-
Retinal is a derivative of Vitamin (C).
-
Rhodopsin is the purplish red protein present in rods only.
-
Retinal is the light absorbing portion of visual photo pigment.
-
-
7) Which one of the following statements is correct?
-
Mango is a parthenocarpic fruit.
-
A proteinaceous aleurone layer is present in maize grain.
-
A sterile pistil is called a staminode.
-
The seed in grasses is not endospermic.
-
-
8) Pollen tablets are available in the market for:
-
Breeding programmes
-
Supplementing food
-
Ex situ conservation
-
In vitro fertilisation
-
-
9) Select the correct option:
-
-
10) The organisation which publishes the Red List of species is:
-
IUCN
-
UNEP
-
WWF
-
ICFRE
-
-
11) A human female with Turner’s syndrome:
-
Has one additional X chromosome.
-
Exhibits male characters.
-
Is able to produce children with normal husband.
-
Has 45 chromosomes with XO.
-
-
12) Match the following and select the correct answer:
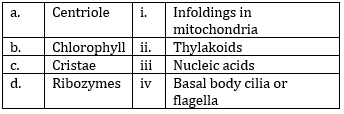
-
-
13) Approximately seventy percent of carbon dioxide absorbed by the blood will be transported to the lungs:
-
In the form of dissolved gas molecules
-
By binding to RBC
-
As carbamino haemoglobin
-
As bicarbonate ions
-
-
14) Which vector can clone only a small fragment of DNA?
-
Yeast artificial chromosome
-
Plasmid
-
Cosmid
-
Bacterial artificial chromosome
-
-
15) The zone of atmosphere in which the ozone layer is present is called:
-
Mesosphere
-
Stratosphere
-
Troposphere
-
Ionosphere
-
-
16) Which one of the following fungi contains hallucinogens?
-
Amanita muscaria
-
Neurospora sp.
-
Ustilago sp.
-
Morchella esculenta
-
-
17) A scrubber in the exhaust of a chemical industrial plant removes:
-
Particulate matter of the size 5 micrometer or above
-
Gases like ozone and methane
-
Particulate matter of the size 2.5 micrometer or less
-
Gases like sulphur dioxide
-
-
18) Select the taxon mentioned that represents both marine and fresh water species:
-
Ctenophora
-
Cephalochordata
-
Cnidaria
-
Echinoderms
-
-
19) A good producer of citric acid is
-
Aspergillus
-
Pseudomonas
-
Clostridium
-
Saccharomyces
-
-
20) DNA fragments generated by the restriction endonucleases in a chemical reaction can be separated by
-
Centrifugation
-
Polymerase chain reaction
-
Electrophoresis
-
Restriction mapping
-
-
21) Which of the following is not correctly matched for the organism and its cell wall degrading enzyme?
-
Bacteria - Lysozyme
-
Plant cells - Cellulase
-
Algae - Methylase
-
Fungi - Chitinase
-
-
22) The colonies of recombinant bacteria appear white in contrast to blue colonies of non-recombinant bacteria because of
-
Non-recombinant bacteria containing beta-galactosidase
-
Insertional inactivation of alpha-galactosidase in non-recombinant bacteria
-
Insertional inactivation of alpha-galactosidase in recombinant bacteria
-
Inactivation of glycosidase enzyme in recombinant bacteria
-
-
23) Which of the following are likely to be present in deep sea water?
-
Archaebacteria
-
Eubacteria
-
Blue-green algae
-
Saprophytic fungi
-
-
24) Natural reservoir of phosphorus is
-
Sea water
-
Animal bones
-
Rocks
-
Fossils
-
-
25) Secondary productivity is the rate of formation of new organic matter by
-
Producers
-
Parasites
-
Consumers
-
Decomposers
-
-
26) Which one of the following is not used for ex situ plant conservation?
-
Field gene banks
-
Seed banks
-
Shifting cultivation
-
Botanical gardens
-
-
27) Kyoto protocol was endorsed at
-
COP-3
-
COP -5
-
COP -6
-
COP -4
-
-
28) Which of the following represent maximum number of species among global biodiversity?
-
Algae
-
Lichens
-
Fungi
-
Mosses and ferns
-
-
29) Match the name of the animal (Column I) with one characteristic (Column II) and the phylum/class (column III) to which it belongs.
-
-
30) Which of the following are correctly matched with respect to their taxonomic classification?
-
Flying fish, cuttlefish, silverfish, -Pisces
-
Centipede, millipede, spider, scorpion-Insecta
-
House fly, butterfly, tsetsefly, silverfish-Insecta
-
Spiny anteater, sea urchin, sea cucumber-Echinodermata
-
-
31) Which group of animals belong to the same phylum?
-
Malarial parasite, Amoeba, Mosquito
-
Earthworm, Pinworm, Tapeworm
-
Prawn, Scorpion, Locusta
-
Sponge, sea anemone, Starfish
-
-
32) One of representatives of Phylum Arthropoda is
-
Cuttlefish
-
Silverfish
-
Pufferfish
-
Flying fish
-
-
33) The H-zone in the skeletal muscle fibres is due to
-
The absence of myofibrils in the central portion of A-band
-
The central gap between myosin filaments in the A-band
-
The central gap between actin filaments extending through myosin filaments in the A-band
-
Extension of myosin filaments in the central portion of the A-band
-
-
34) What external changes are visible ‘after the last moult of a cockroach nymph?
-
Mandibles become harder
-
Anal cerci develop
-
Both forewings and hindwings develop
-
Labium develops
-
-
35) The Golgi complex plays a major role
-
In trapping light and transforming it into chemical energy
-
In digesting proteins and carbohydrates
-
As energy transferring organelles
-
In post translational modification of proteins and glycosidation of lipids
-
-
36) Which one of the following organelles in the figure correctly matches with its function?
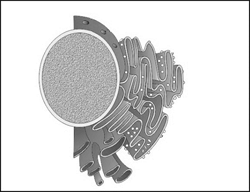
-
Rough endoplasmic reticulum, formation of glycoproteins
-
Golgi apparatus, proteins synthesis
-
Golgi apparatus, formation of glycolipids
-
Rough endoplasmic reticulum, protein synthesis
-
-
37) Which one of the following areas in India, is a hot spot of biodiversity?
-
Sunderbans
-
Western Ghats
-
Eastern Ghats
-
Gangetic plain
-
-
38) Which one of the following is not a functional unit of ecosystem:-
-
Productivity
-
Stratification
-
Energy flow
-
Decomposition
-
-
39) The upright pyramid of number is absent in:-
-
Lake
-
Grassland
-
Pond
-
Forest
-
-
40) Which one of the following is not a gaseous biogeochemical cycle in ecosystem?
-
Nitrogen cycle
-
Carbon cycle
-
Oxygen cycle
-
Phosphorus cycle
-
-
41) Which one of the following is a wrong statement?
-
Greenhouse effect is a natural phenomenon.
-
Eutrophication is a natural phenomenon in freshwater bodies.
-
Most of the forests have been lost in tropical areas.
-
Ozone in upper part of the atmosphere is harmful to animals.
-
-
42) The highest number of species in the world is represented by:-
-
Algae
-
Lichens
-
Fungi
-
Mosses
-
-
43) Yeast is used in the production of:
-
Bread and beer
-
Cheese and butter
-
Citric acid and lactic acid
-
Lipase and pectinase
-
-
44) Which one of the following microbes forms symbiotic association with plants and helps them in their nutrition?
-
Glomus
-
Trichoderma
-
Azotobacter
-
Aspergillus
-
-
45) A single strand of nucleic acid tagged with a radioactive molecule is called:
-
Plasmid
-
Probe
-
Vector
-
Selectable marker
-
-
46) A patient brought to a hospital with myocardial infarction is normally immediately given:
-
Cyclosporin-A
-
Statins
-
Penicillin
-
Streptokinase
-
-
47) A nitrogen fixing microbe associated with Azolla in rice-fields is:
-
Frankia
-
Tolypothrix
-
Spirulina
-
Anabaena
-
-
48) Which one is true statement regarding DNA polymerase used in PCR?
-
It is isolated from a virus.
-
It remains active at high temperature.
-
It is used to ligate introduced DNA in recipient cells.
-
It serves as a selectable marker.
-
-
49) Consumption of which one of the following foods can prevent the kind of blindness associated with vitamin ‘A’ deficiency?
-
Golden rice
-
Bt-brinjal
-
Flavr Savr tomato
-
Canolla
-
-
50) Which one of the following is a case of wrong matching?
-
Micropropagation – In vitro production of plants in large numbers
-
Callus – Unorganised mass of cells produced in tissue culture
-
Somatic hybridisation – Fusion of two diverse cells
-
Vector DNA – Site for t-RNA synthesis
-
-
51) Which part would be most suitable for raising virus free plants for micropropagation?
-
Meristem
-
Node
-
Bark
-
Vascular tissue
-
-
52) For transformation micro-particles coated with DNA to be bombarded with gene are made up of:
-
Silicon or platinum
-
Gold or tungsten
-
Silver or platinum
-
Platinum or zinc
-
-
53) The cyanobacteria are also referred to as:
-
Slime moulds
-
Blue green algae
-
Protists
-
Golden algae
-
-
54) Which one single organism or a pair of organisms is correctly assigned to its/their named taxonomic group?
-
Yeast used in making bread and beer is a fungus.
-
Nostoc and Anabaena are examples of protists.
-
Paramoecium and Plasmodium belong to the same kingdom as that of Penicilium.
-
Lichen is a composite organism formed from the symbiotic associated of an algae and a protozoan.
-
-
55) The rate of formation of new organic matter by rabbit in a grassland is called
-
Gross primary productivity
-
Net productivity
-
Secondary productivity
-
Net primary productivity
-
-
56) Which one of the following organisms is scientifically correctly named, correctly printed according to the International Rules of Nomenclature and correctly described?
-
E.coli – Full name Entamoeba coli, a commonly occurring bacterium in human intestine.
-
Musca domestica – The common house lizard, a reptile.
-
Plasmodium falciparum – A protozoan pathogen causing the most serious type of malaria.
-
Felis tigris – The Indian tiger, well protected in Gir forests.
-
-
57) Which one of the following represents palindromic sequence in DNA?
-
5´ - GATACC - 3´ 3´ - CCTAAG - 5´
-
5´ - GAATTC - 3´ 3´ - CTTAAG - 5´
-
5´ - CCAATG - 3´ 5´ - CATTAG - 3´
-
3´ - GAATCC - 5´ 3´ - GATAAC - 5´
-
-
58) Vernalisation stimulates flowering in
-
Ginger
-
Zaminkand
-
Turmeric
-
Carrot
-
-
59) Which one of the following statements is correct with respect to immunity?
-
Rejection of a kidney graft is the function of B-lymphocytes.
-
Preformed antibodies need to be injected to treat the bite by a viper snake.
-
The antibodies against small pox pathogen are produced by T-lymphocytes.
-
Antibodies are protein molecules, each of which has four light chains.
-
-
60) Which one of the following sets of items in the options A-D are correctly categorised with one exception in it?
-
-
61) Which one of the following pairs is wrongly matched?
-
Mustard – Synergids
-
Ginkgo – Archegonia
-
Salvinia – Prothallus
-
Viroids – RNA
-
-
62) Which one of the following is a wrong statement regarding mutations?
-
Change in a single base pair of DNA does not cause mutation.
-
Deletion and insertion of base pairs cause frame-shift mutations.
-
Cancer cells commonly show chromosomal aberrations.
-
UV and gamma rays are mutagens.
-
-
63) Read the following four statements (A – D).
- Both, photophosphorylation and oxidative phosphorylation involve uphill transport of protons across the membrane.
- In dicot stems, a new cambium originates from the cells of pericycle at the time of secondary growth.
- Stamens in flowers of Gloriosa and Petunia are polyandrous.
- Symbiotic nitrogen-fixers occur in free living state also in soil.
How many of the above statements are right?
-
One
-
Two
-
Three
-
Four
-
64) Where do certain symbiotic microorganisms normally occur in human body?
-
Duodenum
-
Caecum
-
Oral lining and tongue surface
-
Vermiform appendix and rectum
-
-
65) The secretory phase in the human menstrual cycle is also called
-
Follicular phase and lasts for about 13 days
-
Luteal phase and lasts for about 6 days
-
Follicular phase lasting for about 6 days
-
Luteal phase and lasts for about 13 days
-
-
66) Biolistics (gene-gun) is suitable for
-
DNA finger printing
-
Disarming pathogen vectors
-
Transformation of plant cells
-
Constructing recombinant DNA by joining with vectors
-
-
67) A fall in glomerular filtration rate (GFR) activates
-
Posterior pituitary to release vasopressin
-
Juxta glomerular cells to release renin
-
Adrenal cortex to release aldosterone
-
Adrenal medulla to release adrenaline
-
-
68) Represented below is the inheritance pattern of a certain type of trait in humans. Which one of the following conditions could be an example of this pattern?
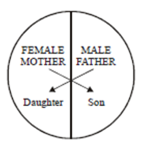
-
Thalassemia
-
Phenylketonuria
-
Sickle cell anaemia
-
Haemophilia
-
-
69) Which one of the following cellular parts is correctly described?
-
Lysosomes - optimally active at a pH of about 8.5
-
Thylakoids - flattened membranous sacs forming the grana of chloroplasts
-
Centrioles - sites for active RNA synthesis
-
Ribosomes - those in chloroplasts are larger (80s) while those in the cytoplasm are smaller (70s)
-
-
70) Which one of the following options gives the correct categorisation of six animals according to the type of nitrogenous wastes (A, B, C), they give out?
-
-
71) Which one of the following characteristics is common both in humans and adult frogs?
-
Ureotelic mode of excretion
-
Four-chambered heart
-
Internal fertilisation
-
Nucleated RBCs
-
-
72) Identify the human developmental stage shown below as well as the related right place of its occurrence in a normal pregnant woman, and select the right option for the two together.

-
-
73) In which one of the following the genus name, its two characters and its class/phylum are correctly matched?
-
-
74) Which one of the following groups of animals is correctly matched with its one characteristic feature without even a single exception?
-
Mammalia: Give birth to young ones
-
Reptilia: Possess 3-chambered heart with one incompletely divided ventricle
-
Chordata: Possess a mouth provided with an upper and a lower jaw
-
Chondrichthyes: Possess cartilaginous endoskeleton
-
-
75) What will you look for to identify the sex of the following?
-
Male shark - Claspers borne on pelvic fins
-
Female Ascaris - Sharply curved posterior end
-
Male frog - A copulatory pad on the first digit of the hindlimb
-
Female cockroach - Anal cerci
-
-
76) The curve given below shows enzymatic activity with relation to three conditions (pH, temperature and substrate concentration). What do the two axes (x and y) represent?
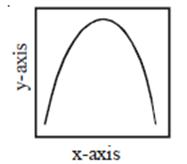
-
-
77) The ciliated columnar epithelial cells in humans are known to occur in
-
Fallopian tubes and urethra
-
Eustachian tube and stomach lining
-
Bronchioles and fallopian tubes
-
Bile duct and oesophagus
-
-
78) Select the correct option with respect to mitosis.
-
Chromosomes move to the spindle equator and get aligned along the equatorial plate in metaphase.
-
Chromatids separate but remain in the centre of the cell in anaphase.
-
Chromatids start moving towards opposite poles in telophase.
-
Golgi complex and endoplasmic reticulum are still visible at the end of prophase.
-
-
79) Which one of the following structural formulae of two organic compounds is correctly identified along with its related function?
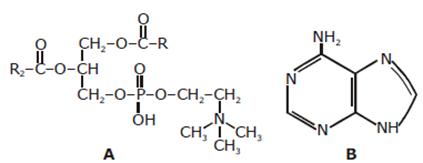
-
A: Lecithin - a component of cell membrane
-
B: Adenine - a nucleotide that makes up nucleic acids
-
A: Triglyceride – a major source of energy
-
B: Uracil - a component of DNA
-
-
80) What was the most significant trend in the evolution of modern man (Homo sapiens) from his ancestors?
-
Increasing brain capacity
-
Upright posture
-
Shortening of jaws
-
Binocular vision
-
-
81) Which one of the following conditions correctly describes the manner of determining the sex in the given example?
-
Homozygous sex chromosomes (XX) produce male in Drosophila.
-
Homozygous sex chromosomes (ZZ) determine female sex in birds.
-
XO type of sex chromosomes determine male sex in grasshopper.
-
XO condition in humans as found in Turner Syndrome, determines female sex.
-
-
82) A person with unknown blood group under ABO system, has suffered much blood loss in an accident and needs immediate blood transfusion.
One of his friends who has a valid certificate of his own blood type, offers for blood donation without delay. What would have been the type of blood group of the donor friend?
-
Type A
-
Type B
-
Type AB
-
Type O
-
-
83) What are those structures that appear as 'beads-on-string' in the chromosomes when viewed under electron microscope?
-
Base pairs
-
Genes
-
Nucleotides
-
Nucleosomes
-
-
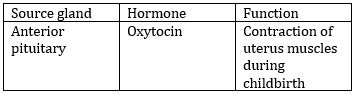
84) Match the source gland with its respective hormone as well as function.
-
-
85) Which of the following is correctly stated as happens in common cockroach?
-
The food is ground by mandibles and gizzard.
-
Malpighian tubules are excretory organs projecting out from the colon.
-
Oxygen is transported by haemoglobin in blood.
-
Nitrogenous excretory product is urea.
-
-
86) A large proportion of oxygen is left unused in the human blood even after its uptake by the body tissues. This O2
-
Helps in releasing more O2 to the epithelium tissues
-
Acts as a reserve during muscular exercise
-
Raises the pCO2 of blood to 75 mm of Hg
-
Is enough to keep oxyhaemoglobin saturation at 96%
-
-
87) Which one of the following enzymes carries on the initial step in the digestion of milk in humans?
-
Trypsin
-
Pepsin
-
Rennin
-
Lipase
-
-
88) Which one of the following is not a part of a renal pyramid?
-
Loops of Henle
-
Peritubular capillaries
-
Convoluted tubules
-
Collecting ducts
-
-
89) One very special feature in the earthworm Pheretima is that
-
It has a long dorsal tubular heart.
-
Fertilisation of eggs occurs inside the body.
-
The typhlosole greatly increases the effective absorption area of the digested food in the intestine.
-
The S-shaped setae embedded in the integument are the defensive weapons used against the enemies.
-
-
90) Two friends are eating together on a dining table. One of them suddenly starts coughing while swallowing some food. This coughing would have been due to improper movement of
-
Tongue
-
Epiglottis
-
Diaphragm
-
Neck
-
-
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
-
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
-
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
-
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
-
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
-
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- 83
- 84
- 85
- 86
- 87
- 88
- 89
- 90