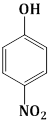
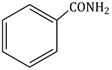
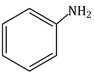
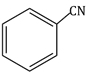
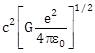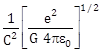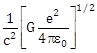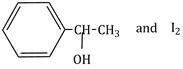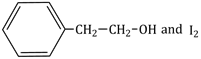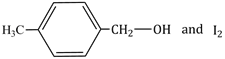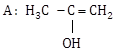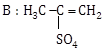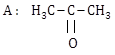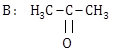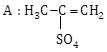Question Paper (Section wise)
-
1) The volume (V) of a monatomic gas varies with its temperature (T), as shown in the graph. The ratio of work done by the gas, to the heat absorbed by it, when it undergoes a change from state A to state B, is.

-
-
2) The fundamental frequency in an open organ pipe is equal to the third harmonic of a closed organ pipe if the length of the closed organ pipe is 20 cm, the length of the open organ pipe is
-
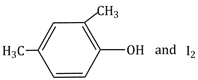
12.5 cm
-
8 cm
-
13.2 cm
-
16 cm
-
-
3) The efficiency of an ideal heat engine working between the freezing point and boiling point of water, is
-
6.25%
-
20%
-
26.8%
-
12.5%
-
-
4) At what temperature will the rms speed of oxygen molecules become just sufficient for escape from the Earth's atmosphere? (Given: Mass of oxygen molecule (m) = 2.76 × 10-26kg
-
5.016 × 104 K
-
8.360 × 104 K
-
2.508 × 104 K
-
1.254 × 104 K
-
-
5) Unpolarised light is incident from air on a plane surface of a material of refractive index 'μ'. At a particular angle of incidence 'i', it is found that the reflected and refracted rays are perpendicular to each other, which of the following options is correct for this situation?
-
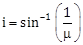
-
Reflected light is polarised with its electric vector perpendicular to the plane of incidence
-
Reflected light is polarised with its electric vector parallel to the plane of incidence
-
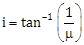
-
-
6) In Young's double slit experiment the separation d between the slits is 2mm, the wavelength λ of the light used is 5896 Å and distance D between the screen and slits is 100 cm. It is found that the angular width of the fringes is 0.20°. To increase the fringe angular width to 0.21° (with same λ and D) the separation between the slits needs to be changed to
-
2.1 mm
-
1.9 mm
-
1.8 mm
-
1.7 mm
-
-
7) An astronomical refracting telescope will have large angular magnification and high angular resolution, when it has an objective lens of
-
large focal length and large diameter
-
large focal length and small diameter
-
small focal length and large diameter
-
small focal length and small diameter
-
-
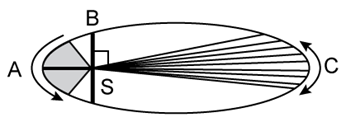
8) The kinetic energies of a planet in an elliptical orbit about the Sun, at positions A, B and C are KA, KB and KC, respectively. AC is the major axis and SB is perpendicular to AC at the position of the Sun S as shown in the figure. Then
-
KB < KA < KC
-
KA > KB > KC
-
KA < KB < KC
-
KB > KA > KC
-
-
9) A solid sphere is in rolling motion. In rolling motion a body possesses translational kinetic energy (Kt) as well as rotational kinetic energy (Kr) simultaneously. The ratio Kt: (Kt + Kr) for the sphere is
-
10:7
-
5:7
-
7:10
-
2:5
-
-
10) Spring of force constant k is cut into length of ratio 1:2:3. They are connected in series and the new force constant is K’. Then they are connected in parallel and force constant is k”. then k’: k” is:
-
1 : 9
-
1 : 11
-
1 : 14
-
1 : 6
-
-
11) The ratio of resolving powers of an optical microscope for two wavelenght λ1 =4000 Å and λ2 =6000 Å is:
-
9: 4
-
3 : 2
-
16 : 81
-
8 : 27
-
-
12) The two nearest harmonics of a tube closed at one end and open at other end are 220 Hz and 260 Hz. What is the fundamental frequency of the system?
-
20 Hz
-
30 Hz
-
40 Hz
-
10 Hz
-
-
13) Consider a drop of rain water having mass 1g falling from a height of 1 km. It hits the ground with a speed of 50 m/s. Take ‘g’ constant with a value 10 m/s2. The work done by the
- gravitational force and the
- resistive force of air is:
-
(i) 1.25 J (ii)-8.25 J
-
(i) 100 J (ii) 8.75 J
-
(i) 10 J (ii)-8.75 J
-
(i) -10 J (ii) -8.25 J
-
14) A physical quantity of the dimension of length that can be formed out of c, G and
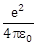 is [c is velocity of light, G is universal constant of gravitation and e is charge]:
is [c is velocity of light, G is universal constant of gravitation and e is charge]: -
-
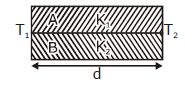
15) Two rods A and B of different materials are welded together as shown in figure. Their thermal conductivities are K1 and K2. The thermal conductivity of the composite rod will be:
-
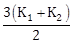
-
K1 + K2
-
2(K1 + K2)
-

-
-
16) A capacitor is charged by a battery. The battery is removed and another identical uncharged capacitor is connected in parallel. The total electrostatic energy of resulting system:
-
decreases by a factor of 2
-
remains the same
-
increases by a factor of 2
-
increases by a factor of 4
-
-
17) In a common emitter transistor amplifier the audio signal voltage across the collector is 3 V. The resistance of collector is 3 KΩ If current gain is 100 and the base resistance is 2 KΩ, the voltage and power gain of the amplifier is:
-
15 and 200
-
150 and 15000
-
20 and 2000
-
200 and 1000
-
-
18) Thermodynamic process are indicated in the following diagram.


-
P→c, Q→a, R→d, S→b
-
P→c, Q→d , R→b, S→a
-
P→d, Q→b, R→a, S→c
-
P→a, Q→c, R→d, S→b
-
-
19) A person can see clearly objects only when they lie between 50 cm and 400 cm from his eyes. In order to increase the maximum distance of distinct vision to infinity, the type and power of the correcting lens, the person has to use, will be
-
convex, + 0.15 diopter
-
convex, + 2.25 diopter
-
convex, - 0.25 diopter
-
convex, -0.2 diopter
-
-
20) A linear aperture whose width is 0.02 cm is placed immediately in front of a lens of focal length 60 cm. The aperture is illuminated normally by a parallel beam of wavelength 5 × 10-5 cm. The distance of the first dark band of the diffraction pattern from the center of the screen is
-
0.15
-
0.10
-
0.25
-
0.20
-
-
21) Electron of mass m with de – Broglie wavelength λ fall on the target in an X – ray tube. The cutoff wavelength (λ0) of the emitted X – ray is
-
-
22) Photons with energy 5 eV are incident on a cathode C in a photoelectric cell. The maximum energy of emitted photoelectrons is 2 eV. When photons of energy 6 eV are incident on C, no photoelectrons will reach the anode A, if the stopping potential of A relative to C is
-
-3 V
-
+3 V
-
+4 V
-
-1 V
-
-
23) If an electron in a hydrogen atoms jumps from the 3 rd orbit to the 2 nd orbit, it emits a photo of wavelength λ. When it jumps from he 4th orbit to the 3rd orbit, the corresponding wavelength of the photon will be
-
-
24) The half – life of a radioactive substance is 30 minutes. The time (in minutes) taken between 40% decay and 85 % decay of the same radioactive substance is
-
60
-
15
-
30
-
45
-
-
25) For CE transistor amplifier. The audio signal voltage across the collector resistance of 2kΩ is 4 v. if the current amplification factor of the transistor is 100 and the base resistance is 1 kΩ, then the input signal voltage is
-
15 mV
-
10 mV
-
20 mV
-
30 mV
-
-
26) The given circuit has two ideal diodes connected as shown in the figure below. The current flowing through the resistance R1 will be

-
3.13 A
-
2.5 A
-
10.0 A
-
1.43 A
-
-
27) What is the output Y in the following circuit when all the three inputs A, B, C are first 0 and then 1?
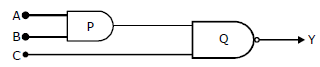
-
1, 1
-
0, 1
-
0, 0
-
1, 0
-
-
28) From a disc of a radius R and mass M, a circular hole of diameter R, whose rim passes through the centre is cut. What is the moment of inertia of the remaining part of the disc about a perpendicular axis, passing through the centre?
-
15 MR2/32
-
13 MR2/32
-
11 MR2/32
-
9 MR2/32
-
-
29) A square loop ABCD carrying a current i, is placed near and coplanar with a long straight conductor XY, carrying a current I, the net force on the loop will be:
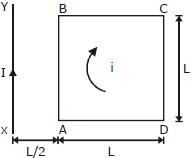
-
-
30) The magnetic susceptibility is negative for:
-
diamagnetic material only
-
paramagnetic material only
-
ferromagnetic material only
-
paramagnetic and ferromagnetic materials
-
-
31) A siren emitting a sound of frequency 800 Hz moves away from an observer towards a cliff at a speed of 15 ms-1. Then, the frequency of sound that the observer hears in the echo reflected from the cliff is: (Take velocity of sound in air = 330 ms-1)
-
765 Hz
-
800 Hz
-
838 Hz
-
885 Hz
-
-
32) A capacitor of 2 μF is charged as shown in the diagram. When the switch S is turned to position 2, the percentage of its stored energy dissipated is:
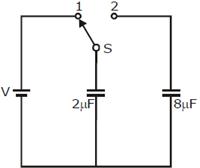
-
0%
-
20%
-
75%
-
80%
-
-
33) In a diffraction pattern due to a single slit of width ‘a’, the first minimum is observed at an angle 30° when light of wavelength 5000Å is incident on the slit. The first secondary maximum is observed at an angle of:
-
-
34) At what height from the surface of earth the gravitation potential and the value of g are –5.4 × 107J kg-2 and 6.0 ms-2 respectively? Take the radius of earth as 6400 km.
-
2600 km
-
1600 km
-
1400 km
-
2000 km
-
-
35) Out of the following options which one can be used to produce a propagating electromagnetic wave?
-
A charge moving at constant velocity.
-
A stationary charge
-
A chargeless particle
-
An accelerating charge
-
-
36) Two identical charged spheres suspended from a common point by two massless strings of lengths l, are initially at a distance d (d << l) apart because of their mutual repulsion. The charges begin to leak from both the spheres at a constant rate. As a result, the spheres approach each other with a velocity ν. Then ν varies as a function of the distance x between the spheres, as:
-
-
37) The cylindrical tube of a spray pump has radius R, one end of which has n fine holes, each of radius r. If the speed of the liquid in the tube is V, the speed of the ejection of the liquid through the holes is-
-
-
38) Point masses m1 and m2 are placed at the opposite ends of a rigid rod of length L, and negligible mass. The rod is to be set rotating about an axis perpendicular to it. The position of point P on this rod through which the axis should pass so that the work required to set the rod rotating with angular velocity ɷ0 is minimum, is given by:

-
-
39) A proton and an alpha particle both enter a region of uniform magnetic field B, if the radius of circular orbits for both the particles is equal and the kinetic energy acquired by proton is 1 MeV, the energy acquired by the alpha particle will be-
-
4 MeV
-
0.5 MeV
-
1.5 MeV
-
1 MeV
-
-
40) A plank with a box on it at one end is gradually raised about the other end. As the angle of inclination with the horizontal reaches 30°, the box starts to slip and slides 4.0 m down the plank in 4.0 s. The coefficients of static and kinetic friction between the box and the plank will be, respectively-

-
0.6 and 0.6
-
0.6 and 0.5
-
0.5 and 0.6
-
0.4 and 0.3
-
-
41) An ideal gas is compressed to half its initial volume by means of several processes. Which of the process results in the maximum work done on the gas?
-
Adiabatic
-
Isobaric
-
Isochoric
-
Isothermal
-
-
42) A ball is thrown vertically downwards from a height of 20 m with an initial velocity υ0. It collides with the ground, losses 50 percent of its energy in collision and rebounds to the same height. The initial velocity υ0 is (take g = 10 ms-2)
-
14 ms-1
-
20 ms-1
-
28 ms-1
-
10 ms-1
-
-
43) In the spectrum of hydrogen, the ratio of the longest wavelength in the Lyman series to the longest wavelength in the blamer series is-
-
-
44) A source of sound S emitting waves of frequency 100 Hz and an observer O are located at some distance from each other. The source is moving with a speed of 19.4 ms-1 at an angle of 60° with the source observer line as shown in the figure. The observer is at rest. The apparent frequency observer by the observer (velocity of sound in air 330 ms-1) is-
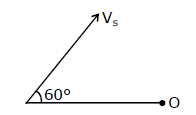
-
100 Hz
-
103 Hz
-
106 Hz
-
97 Hz
-
-
45) If dimensions of critical velocity vc od a liquid flowing through a tube are expressed as
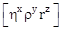 , where η , ρ and r are the coefficient of viscosity of liquid, density of liquid and radius of the tube respectively, then the values of x, y and z are given by-
, where η , ρ and r are the coefficient of viscosity of liquid, density of liquid and radius of the tube respectively, then the values of x, y and z are given by--
1, -1, -1
-
-1, -1, 1
-
-1, -1, -1
-
1,1,1
-
-
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
-
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
-
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
-
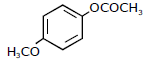
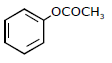
1) Following solutions were prepared by mixing different volumes of NaOH and HCI of different concentration:
a.

b.

c.
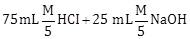
d.
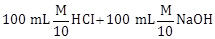
pH of which one of them will be equal to 1?
-
d
-
a
-
b
-
c
-
-
2) On which of the following properties does the coagulating power of an ion depend?
-
Both magnitude and sign of the charge on the ion
-
Size of the ion alone
-
The magnitude of the charge on the ion alone
-
The sign of charge on the ion alone
-
-
3) The solubility of BaSO4 in water is 2.42 × 10-3 gL-1 at 298 K. The value of its solubility product (Ksp) will be (Given molar mass of BaSO4 = 233 g mol-1)
-
1.08 × 10-14 mol2L-2
-
1.08 × 10-12 mol2L-2
-
1.08 × 10-10 mol2L-2
-
1.08 × 10-8 mol2L-2
-
-
4) Given van der Waals constant for NH3, H2, O2 and CO2 are respectively 4.17, 0.244, 1.36 and 3.59, which one of the following gases in most easily liquefied?
-
O2
-
H2
-
NH3
-
CO2
-
-
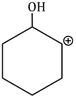
5) In the reaction
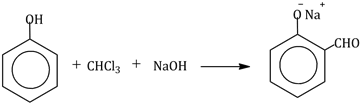
The electrophile involved is
-
dichloromethyl anion
-
formyl cation (CHO)
-
dichloromethyl cation
-
dichlorocarbene (:CCl2)
-
-
6) Carboxylic acids have higher boiling points than aldehydes, ketones and even alchohols of comparable molecular mass. It is due to their
-
more extensive association of carboxylic acid via van der Waals force of attraction
-
formation of carboxylate ion
-
formation of intramolecular H-bonding
-
formation of intermolecular H- bonding
-
-
7) Compound A, C8 H10 O, is found to react with NaOI (produced by reacting Y with NaOH) and yields a yellow precipitate with characteristic smell.
A and Y are respectively
-
-
8) Magnesium react with an element (X) to from an ionic compound. If the ground sate electronic configuration of (X) is 1s2 2s22p3, the simplest formula for this compound is
-
Mg2X
-
MgX2
-
Mg2 X3
-
Mg3 X2
-
-
9) Iron exhibits bcc structure at room temperature. Above 900° C, it transforms to fcc structure. The ratio for density of iron at room temperature to that at 900° C (assuming molar mass and atomic radii of iron remains constant with temperature) is
-
-
10) Name the gas that can readily decolourise acidified KMnO4 solution:
-
SO2
-
NO2
-
P2O5
-
CO2
-
-
11) Mechanism of a hypothetical reaction X2 + Y2→ 2 XY is given below:
- X2→ X + X (fast)
- X + Y2⇌ XY + Y (slow)
- X2 + Y2→ XY (fast)
The overall order of the reaction will be:
-
2
-
0
-
1.5
-
1
-
12) The element Z = 114 has been discovered recently. It will belong to which of the following family/group and electronic configuration?
-
Carbon family, [Rn] 5f14 6d10 7s2 7p2
-
Oxygen family, [Rn] 5f14 6d10 7s2 7p4
-
Nitrogen family, [Rn] 5f14 6d10 7s2 7p6
-
Halogen family, [Rn] 5f14 6d10 7s2 7p5
-
-
13) The heating of phenyl-methyl ethers with HI produces-
-
Iodobenzene
-
Phenol
-
Benezene
-
Ethyl Chlorides
-
-
14) Which one is the correct order of acidity?
-
CH≡CH > CH3–C≡CH > CH2 = CH2 > CH3 – CH3
-
CH ≡CH>CH2=CH2 > CH3–C ≡ CH > CH3–CH3
-
CH3–CH3 > CH2=CH2 > CH3–C ≡ CH > CH ≡ CH
-
CH2=CH2 > CH3–CH=CH2 > CH3–C ≡ CH > CH ≡ CH
-
-
15) Predict the correct intermediate and product in the following reaction:
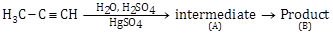
-
-
16) The equilibrium constant of the following are:
N2 + 3 H2⇌ 2 NH3 K1
N2 + O2⇌ 2 NO K2

The equilibrium constant (K) of the reaction:
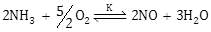 , will be:
, will be: -
-
17) Which one is the most acidic compound?
-
-
18) The correct increasing order of basic strength for the following compound is:
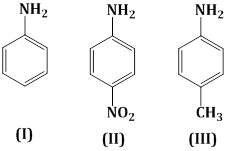
-
III < I < II
-
III < II < I
-
II < I <III
-
II < III < I
-
-
19) A given nitrogen-containing aromatic compound A reacts with Sn/HCl, followed by HNO2 to give an unstable compound B. B, on treatment with phenol, forms a beautiful coloured compound C with the molecular formula C12H10N2O. The structure of compound A is
-
-
20) Consider the reaction

This reaction will be the fastest in
-
Water
-
ethanol
-
methanol
-
N, N’-dimethylformamide(DMF)
-
-
21) The correct structure of the product A formed in the reaction

-
-
22) Which among the given molecules can exhibit tautomerism?
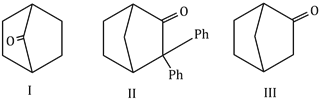
-
Both II and III
-
III only
-
Both I and III
-
Both I and II
-
-
23) The correct order of strengths of the carboxylic acids
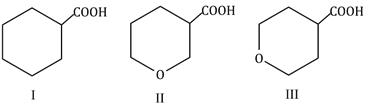
-
II > I > III
-
I > II > III
-
II > III > I
-
III > II > I
-
-
24) The compound that will react most readily with gaseous bromine has the formula
-
C2H4
-
C3H6
-
C2H2
-
C4H10
-
-
25) Which one of the following compounds shows the presence of intramolecular hydrogen bond?
-
Concentrated acetic acid
-
H2O2
-
HCN
-
Cellulose
-
-
26) The molar conductivity of a 0.5 mol/dm3 solution of AgNO3 with electrolytic conductivity of 5.76 × 10–3 S cm–1 at 298 K is
-
28.8 S cm2/mol
-
2.88 S cm2/mol
-
11.52 S cm2/mol
-
0.086 S cm2/mol
-
-
27) The decomposition of phosphine (PH3) on tungsten at low pressure is a first order reaction. It is because the
-
rate of decomposition is very slow
-
rate is proportional to the surface coverage
-
rate is inversely proportional to the surface coverage
-
rate is independent of the surface coverage
-
-
28) Consider the molecules CH4 NH3 and H2O. Which of the given statements is false?
-
The H – C – H bond angle in CH4, the H –N – H bond angle in NH3, and the H– O – H bond angle in H2O are all greater than 90°.
-
The H – O – H bond angle in H2O is larger than the H – C – H bond angle in CH4
-
The H – O – H bond angle in H2 O is smaller than the H – N – H bond angle in NH3
-
The H – C – H bond angle in CH4 is larger than the H – N – H bond angle in NH3
-
-
29) In the reaction
-
X = 1- Butyne ; Y = 3- Hexyne
-
X = 2- Butyne ; Y = 3- Hexyne
-
X = 2- Butyne ; Y = 2- Hexyne
-
X = 1- Butyne ; Y = 2- Hexyne
-
-
30) Among the following the correct order of acidity is:
-
HCIO3 <HCIO4 < HCIO2 < HCIO
-
HCIO < HCIO2 < HCIO3 < HCIO4
-
HCIO2 < HCIO < HCIO3 < HCIO4
-
HCIO4 < HCIO2 < HCIO < HCIO3
-
-
31) The rate of first-order reaction is 0.04 mol l−1 S−1 at 10 second and 0.03 mol l-1 S-1 at 20 second after initiation of the reaction. The half-life period of the reaction is:
-
24.1 s
-
34.1 s
-
44.1 s
-
54.1 s
-
-
32) Which one of the following characteristics is associated with adsorption?
-
∆G is negative but ∆H and ∆S are positive
-
ΔG, ΔH and ΔS all the negative
-
∆G and ∆H are negative but ∆S is positive
-
∆G and ∆S are negative but ∆H is positive
-
-
33) In which of the following options the order of arrangement does not agree with the variation of property indicated against it?
-
Al3+ < Mg2 < Na+ < F− (Increasing ionic size)
-
B < C < O < N (Increasing first ionization enthalpy)
-
I < Br < Cl < F (Increasing electron gain enthalpy)
-
Li < Na < k < Rb (Increasing metallic radius)
-
-
34) Which of the following statements is false?
-
Mg2+ ions from a complex with ATP
-
Ca2+ ions are important in blood clotting
-
Ca2+ ions are not important in maintaining the regular beating of the heart
-
Mg2+ ions are important in the green parts of plants
-
-
35) Which of the following statements about hydrogen is incorrect?
-
Hydrogen has three isotopes of which tritium is the most common
-
Hydrogen never acts as cation in ionic salts
-
Hydronium ion, H3O+ exists freely in solution
-
Dihydrogen acts as a reducing agent
-
-
36) The correct statement regarding a carbonyl compound with a hydrogen atom on its alphacarbon is:
-
a carbonyl compound with a hydrogen atom on its alpha-carbon never equilibrates with its corresponding enol.
-
a carbonyl compound with a hydrogen atom on its alpha-carbon rapidly equilibrates with its corresponding enol and this process is known as aldehyde-ketone equilibration.
-
a carbonyl compound with a hydrogen atom on its alpha-carbon rapidly equilibrates with its corresponding enol and this process is known as carbonylation.
-
a carbonyl compound with a hydrogen atom on its alpha-carbon rapidly equilibrates with its corresponding enol and this process is known as keto-enol tautomerism.
-
-
37) In which of the following pairs, both the species are not isostructural
-
XeF4, XeO4
-
SiCl4, PCl4 +
-
diamond, silicon carbide
-
NH3, PH3
-
-
38) Which one of the following esters gets hydrolysed most easily under alkaline conditions?
-
-
39) Reaction of phenol with chloroform in presence of dilute sodium hydroxide finally introduces which one of the following functional group?
-
-CHO
-
-CH2Cl
-
-COOH
-
-CHCl2
-
-
40) Which of the following reactions can be used for the preparation of alkyl haldies?

-
III and IV only
-
I, III and IV only
-
I and II only
-
IV only
-
-
41) In an SN1 reaction on chiral centres, there is:
-
100 % inversion
-
100 % racemization
-
inversion more than retention leading to partial racemization
-
100 % retention
-
-
42) Which of the following is not product of dehydration of
-
-
43) On heating which of the following release CO2 most easily?
-
CaCO3
-
K2CO3
-
Na2CO3
-
MgCO3
-
-
44) In the reaction with HCl, an alkene reacts in accordance with the Markovnikov’s rule, to give a product 1 – chloro – 1 – methylcyclohexane. The possible alkene is:
-

-
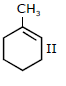
-
I and II
-
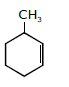
-
-
45) Number of possible isomers for the complex [Co(en)2Cl2]Cl will be : en = ethylenediamine
-
4
-
2
-
1
-
3
-
-
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
-
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
-
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
-
1) What is the role of NAD+ in cellular respiration?
-
It is a nucleotide source for ATP synthesis.
-
It functions as an electron carrier.
-
It functions as an enzyme.
-
It is the final electron acceptor for anaerobic respiration.
-
-
2) Oxygen is not produced during photosynthesis by
-
Cycas
-
Nostoc
-
Green sulphur bacteria
-
Chara
-
-
3) Double fertilisation is
-
Fusion of two male gametes with one egg
-
Fusion of one male gamete with two polar nuclei
-
Fusion of two male gametes of a pollen tube with two different eggs
-
Syngamy and triple fusion
-
-
4) In which of the following forms is iron adsorbed by plants?
-
Free element
-
Ferrous
-
Ferric
-
Both ferric and ferrous
-
-
5) Which of the following elements is responsible for maintaining turgor in cells?
-
Potassium
-
Sodium
-
Magnesium
-
Calcium
-
-
6) Which one of the following plants shows a very close relationship with a species of moth, where none of the two can complete, its life cycle without the other?
-
Banana
-
Yucca
-
Hydrilla
-
Viola
-
-
7) Pollen grains can be stored for several years in liquid nitrogen having temperature of
-
-196°C
-
-80°C
-
-120°C
-
-160°C
-
-
8) What type of ecological pyramid would be obtained with the following data?
Secondary consumer: 120 g
Primary consumer: 60 g
Primary producer: 10 g
-
Upright pyramid of numbers
-
Pyramid of energy
-
Inverted pyramid of biomass
-
Upright pyramid of biomass
-
-
9) Natality refers to
-
Number of individuals leaving the habitat
-
Birth rate
-
Death rate
-
Number of individuals entering habitat
-
-
10) World Ozone Day is celebrated on
-
16th September
-
21st April
-
5th June
-
22nd April
-
-
11) In stratosphere, which of the following elements acts as a catalyst in degradation of ozone and release of molecular oxygen?
-
Fe
-
Cl
-
Carbon
-
Oxygen
-
-
12) Niche is
-
The range of temperature that the organism needs to live.
-
The physical space where an organism live.
-
All the biological factors in the organism’s environment.
-
The functional role played by the organism where it lives.
-
-
13) Which of the following is a secondary pollutant?
-
SO2
-
CO2
-
CO
-
O3
-
-
14) Which of the following statement is correct?
-
Horsetails are gymnosperms.
-
Selaginella is heterosporous while Salvinia is homosporous.
-
Ovules are not enclosed by ovary wall in gymnosperms.
-
Stems are usually unbranched in both Cycas and Cedrus.
-
-
15) Pneumatophores occurs in
-
Carnivorous plants
-
Free-floating hydrophytes
-
Halophytes
-
Submerged hydrophytes
-
-
16) Sweet potato is a modified
-
Tap root
-
Adventitious root
-
Stem
-
Rhizome
-
-
17) Secondary xylem and phloem in dicot stem are produced by:
-
Phellogen
-
Vascular cambium
-
Apical meristems
-
Axillary meristems
-
-
18) Select the wrong statement.
-
Pseudopodia are locomotory and feeding structures in sporozoans.
-
Mushrooms belong to Basidiomycetes.
-
Cell wall is present in members of Fungi and Plantae.
-
Mitochondria are the powerhouse of the cell in all kingdoms except Monera.
-
-
19) Which one of the following statements is correct with reference to enzymes?
-
Holoenzyme = Apoenzyme + Coenzyme
-
Coenzyme = Apoenzyme + Holoenzyme
-
Holoenzyme = Coenzyme + Co-factor
-
Apoenzyme = Holoenzyme + Coenzyme
-
-
20) A decrease in blood pressure/volume will not cause the release of:
-
Atrial Natriuretic Factor
-
Aldosterone
-
ADH
-
Renin
-
-
21) Which cells of 'Crypts of Lieberkuhn' secrete antibacterial lysozyme?
-
Paneth cells
-
Zymogen cells
-
Kupffer cells
-
Argentaffin cells
-
-
22) Which of the following are not polymeric?
-
Proteins
-
Polysaccharides
-
Lipids
-
Nucleic acids
-
-
23) Functional megaspore in an angiosperm develops into:
-
Endosperm
-
Embryo sac
-
Embryo
-
Ovule
-
-
24) Myelin sheath is produced by:
-
Astrocytes and Schwann cells
-
Oligodendrocytes and osteoclasts
-
Osteoclasts and astrocytes
-
Schwann cells and oligodendrocytes
-
-
25) Attractants and rewards are required for:
-
Entomophily
-
Hydrophily
-
Cleistogamy
-
Anemophily
-
-
26) Receptor sites for neurotransmitters are present on the
-
Pre-synaptic membrane
-
Tips of axons
-
Post-synaptic membrane
-
Membranes of synaptic vesicles
-
-
27) Coconut fruit is a:
-
Berry
-
Nut
-
Capsule
-
Drupe
-
-
28) Adult human RBCs are enucleate. Which of following statement(s) provide the most appropriate explanation for this feature?
- They do not need to reproduce.
- They are somatic cells.
- They do not metabolise.
- All their internal space is available for oxygen transport.
-
Only a
-
a, c, and d
-
b and c
-
only d
-
29) Capacitation occurs in:
-
Epididymis
-
Vas deferens
-
Female reproductive tract
-
Rete testis
-
-
30) Which of the following are found in extreme saline conditions?
-
Eubacteria
-
Cyanobacteria
-
Mycobacteria
-
Archaebacteria
-
-
31) Asymptote in a logistic growth curve is obtained when:
-
k = N
-
k > N
-
k < N
-
The value of r approaches zero
-
-
32) Artificial selection to obtain cows yielding higher milk output represents:
-
Directional selection as it pushes the mean of the character in one direction.
-
Disruptive selection as it splits the population into two, one yielding higher output and the other lower output.
-
Stabilising selection followed by disruptive selection as it stabilises the population to produce higher yielding cows.
-
Stabilising selection as it stabilises this character in the population.
-
-
33) Select the mismatch:
-
Rhodospirillum- Mycorrhiza
-
Anabaena - Nitrogen fixer
-
Rhizobium - Alfalfa
-
Frankia - Alnus
-
-
34) Good vision depends on adequate intake of carotene rich food.
Select the best option from the following statements.
- Vitamin A derivatives are formed from carotene.
- The photopigments are embedded in the membrane discs of the inner segment.
- Retinal is a derivative of Vitamin A.
- Retinal is a light absorbing part of all the visual photopigments.
-
a, c and d
-
a and c
-
b, c and d
-
a and b
-
35) The DNA fragments separated on an agarose gel can be visualised after staining with:
-
Acetocarmine
-
Aniline blue
-
Ethidium bromide
-
Bromophenol blue
-
-
36) The hepatic portal vein drains blood to liver from the:
-
Stomach
-
Kidneys
-
Intestine
-
Heart
-
-
37) A foreign DNA and plasmid cut by the same restriction endonuclease can be joined to form a recombinant plasmid using
-
Ligase
-
EcoRI
-
Taq polymerase
-
Polymerase III
-
-
38) Which of the following is not a component of downstream processing?
-
Expression
-
Separation
-
Purification
-
Preservation
-
-
39) Which of the following restriction enzymes produces blunt ends?
-
HindIII
-
SalI
-
EcoRV
-
XhoI
-
-
40) Which kind of therapy was given in 1990 to a four-year-old girl with adenosine deaminase (ADA) deficiency?
-
Radiation therapy
-
Gene therapy
-
Chemotherapy
-
Immunotherapy
-
-
41) How many hot spots of biodiversity in the world have been identified till date by Norman Myers?
-
43
-
17
-
25
-
34
-
-
42) The primary producers of the deep-sea hydrothermal vent ecosystem are
-
Coral reefs
-
Green algae
-
Chemosynthetic bacteria
-
Blue-green algae
-
-
43) Which of the following is correct for r-selected species?
-
Small number of progeny with large size
-
Large number of progeny with small size
-
Large number of progeny with large size
-
Small number of progeny with small size
-
-
44) If ‘+’ sign is assigned to beneficial interaction, ‘-’ sign to detrimental and ‘0 ‘ sign to neutral interaction, then the population interaction represented by ‘ + ‘ ‘ -’ refers to
-
Parasitism
-
Mutualism
-
Amensalism
-
Commensalism
-
-
45) Which of the following is correctly matched?
-
Stratification - Population
-
Aerenchyma - Opuntia
-
Age pyramid - Biome
-
Parthenium hysterophorus - Threat to biodiversity
-
-
46) Red List contains data or information on
-
Marine vertebrates only
-
All economically important plants
-
Plants whose product are in international trade
-
Threatened species
-
-
47) Which one of the following is wrong for fungi?
-
They are both unicellular and multicellular.
-
They are eukaryotic.
-
All fungi possess a purely cellulosic cell wall.
-
They are heterotrophic.
-
-
48) Methanogens belong to
-
Slime moulds
-
Eubacteria
-
Archaebacteria
-
Dinoflagellates
-
-
49) Select the wrong statement.
-
Diatoms are microscopic and float passively in water.
-
The walls of diatoms are easily destructible.
-
Diatomaceous earth is formed by the cell walls of diatoms.
-
Diatoms are chief producers in the oceans.
-
-
50) The label of a herbarium sheet does not carry information on
-
Height of the plant
-
Date of collection
-
Name of collector
-
Local names
-
-
51) Conifers are adapted to tolerate extreme environmental conditions because of presence of
-
Vessels
-
Broad hardy leaves
-
Superficial stomata
-
Thick cuticle
-
-
52) Which one of following statements is wrong?
-
Laminaria and Sargassum are used as food
-
Algae increase the level of dissolved oxygen in the immediate environment.
-
Algin is obtained from red algae, and carrageenan from brown algae.
-
Agar-agar is obtained from Gelidium and Gracilaria.
-
-
53) The term ‘polyadelphous’ is related to
-
Calyx
-
Gynoecium
-
Androecium
-
Corolla
-
-
54) How many plants among Indigofera, Sesbania, Salvia, Allium, Aloe, mustard, groundnut, radish, gram and turnip have stamens with different lengths in their flowers?
-
Six
-
Three
-
Four
-
Five
-
-
55) Gause’s principle of competitive exclusion states that:
-
More abundant species will exclude the less abundant species through competition.
-
Competition for the same resources excludes species having different food preference.
-
No two species can occupy the same niche indefinitely for the same limiting resources.
-
Larger organism exclude smaller ones through competition.
-
-
56) The two polypeptides of human insulin are linked together by:
-
Hydrogen bonds
-
Phosphodiester bond
-
Covalent bond
-
Disulphide bridges
-
-
57) The coconut water from tender coconut represents:
-
Endocarp
-
Fleshy mesocarp
-
Free nuclear proembryo
-
Free nuclear endosperm
-
-
58) Which of the following statements is wrong for viroids?
-
They lack a protein coat.
-
They are smaller than viruses.
-
They cause infections.
-
Their RNA is of high molecular weight.
-
-
59) Which of the following features is not present in the Phylum-Arthropoda?
-
Chitinous exoskeleton
-
Metameric segmentation
-
Parapodia
-
Jointed appendages
-
-
60) Which of the following most appropriately describes haemophilia?
-
Recessive gene disorder
-
X-linked recessive gene disorder
-
Chromosomal disorder
-
Dominant gene disorder
-
-
61) Emerson’s enhancement effect and Red drop have been instrumental in the discovery of:
-
Photophosphorylation and non-cyclic electron transport
-
Two photosystems operating simultaneously
-
Photophosphorylation and cyclic electron transport
-
Oxidative phosphorylation
-
-
62) In which of the following, all three are macronutrients?
-
Boron, zinc, manganese
-
Iron, copper, molybdenum
-
Molybdenum, magnesium, manganese
-
Nitrogen, nickel, phosphorus
-
-
63) Name the chronic respiratory disorder caused mainly by cigarette smoking.
-
Emphysema
-
Asthma
-
Respiratory acidosis
-
Respiratory alkalosis
-
-
64) A system of rotating crops with legume or grass pasture to improve soil structure and fertility is called:
-
Ley farming
-
Contour farming
-
Strip farming
-
Shifting farming
-
-
65) Mitochondria and chloroplast are:
- Semi-autonomous organelles
- Formed by the division of pre-existing organelle and they contain DNA but lack protein synthesising machinery.
Which one of the following options is correct?
-
Both (a) and (b) are correct.
-
(b) is true but (a) is false.
-
(a) is true but (b) is false.
-
Both (a) and (b) are false.
-
66) In the context of amniocentesis, which of the following statement is incorrect?
-
It is usually done when a woman is between 14-16 weeks pregnant.
-
It is used for prenatal sex determination.
-
It can be used for detection of Down’s syndrome.
-
It can be used for detection of cleft palate.
-
-
67) In a chloroplast the highest number of protons are found in:
-
Stroma
-
Lumen of thylakoids
-
Inter membrane space
-
Antennae complex
-
-
68) Photosensitive compound in human eye is made up of:
-
Guanosine and retinol
-
Opsin and retinol
-
Opsin and retinol
-
Transducin and retinene
-
-
69) Spindle fibres attach on to:
-
Telomere of the chromosome
-
Kinetochore of the chromosome
-
Centromere of the chromosome
-
Kinetosome of the chromosome
-
-
70) Which is the National Aquatic Animal of India?
-
Gangetic shark
-
River dolphin
-
Blue whale
-
Sea-horse
-
-
71) Which of the following is required as inducer (s) for the expression of lac operon?
-
Glucose
-
Galactose
-
Lactose
-
Lactose and galactose
-
-
72) Which of the following pairs of hormones are not antagonistic (having opposite effects) to each other?
-
Parathormone – Calcitonin
-
Insulin – Glucagon
-
Aldosterone – Atrial Natriuretic Factor
-
Relaxin - Inhibin
-
-
73) Read the different components from (a) to (d) in the list given below and tell the correct order of the components with reference to their arrangement from outer side to inner side in a woody dicot stem:
(1) Secondary cortex (2) Wood
(3) Secondary phloem (4) Phellem
-
(3), (4), (2), (1)
-
(1), (2), (4), (3)
-
(4), (1), (3), (2)
-
(4), (3), (1), (2)
-
-
74) Chromatophores take part in:
-
Photosynthesis
-
Growth
-
Movement
-
Respiration
-
-
75) Which of the following joints would allow no movement?
-
Fibrous joint
-
Cartilaginous joint
-
Synovial Joint
-
Ball and socket joint
-
-
76) The wheat grain has an embryo with one large, shield-shaped cotyledon known as:
-
Epiblast
-
Coleorhiza
-
Scutellum
-
Coleoptile
-
-
77) A gene showing codominance has:
-
One allele dominant on the other
-
Alleles tightly linked on the same chromosome
-
Alleles that are recessive to each other
-
Both alleles independently expressed in the heterozygote
-
-
78) Which of the following structures is not found in a prokaryotic cell?
-
Nuclear envelope
-
Ribosome
-
Mesosome
-
Plasma membrane
-
-
79) The term "linkage" was coined by:
-
T.H. Morgan
-
T. Boveri
-
G. Mendel
-
W. Slitton
-
-
80) The imperfect fungi which are decomposers of litter and help in mineral cycling belong to
-
Deuteromycetes
-
Basidiomycetes
-
Phycomycetes
-
Ascomvcetes
-
-
81) Match the columns and identify the correct option.

-
-
82) Select the wrong statement.
-
The viroids were discovered by D.J. Ivanowski.
-
W.M. Stanley showed that viruses could be crystallised.
-
The term 'contagium vivum fludum' was coined by M.W. Beijerinck.
-
Mosaic disease in tobacco and AIDS in human beings are caused by viruses.
-
-
83) During biological nitrogen fixation inactivation of nitrogenase by oxygen poisoning is prevented by
-
Leghaemogolobin
-
XanthophyII
-
Carotene
-
Cytochrome
-
-
84) The species confined to a particular region and not found elsewhere is termed as:
-
Keystone
-
Alien
-
Endemic
-
Rare
-
-
85) Which one of the following hormones is not involved in sugar metabolism?
-
Cortisone
-
Aldosterone
-
Insulin
-
Glucagon
-
-
86) Which of the following is not a function of the skeletal system?
-
Production of erythrocvtes
-
Storage of minerals
-
Production of body heat
-
Locomotion
-
-
87) Which one of the following is not applicable to RNA?
-
Complementary base pairing
-
5’ phosphoryl and 3’ hydroxyl ends
-
Heterocyclic nitrogenous bases
-
Chargaff's rule
-
-
88) Which one is a wrong statement?
-
Archegonia are found in Bryophyta, Pteridophyta and Gymnosperms.
-
Mucor has biflagellate zoospores.
-
Haploid endosperm is a typical feature of gymnosperms.
-
Brown algae have chlorophyll a and c, and fucoxanthin.
-
-
89) A childless couple can be assisted to have a child through a technique called GIFT. The full form of this technique is:
-
Gamete Inseminated Fallopian Transfer
-
Gamete Intra Fallopian Transfer
-
Gamete Internal Fertilization and Transfer
-
Germ cell Internal Fallopian Transfer
-
-
90) The wings of a bird and wings of an insect are:
-
Homologous structures and represent divergent evolution.
-
Analogous structures and represent convergent evolution.
-
Phylogenetic structures and represent divergent evolution.
-
Homologous structures and represent convergent evolution.
-
-
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
-
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
-
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
-
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
-
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
-
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- 83
- 84
- 85
- 86
- 87
- 88
- 89
- 90