Question Paper (Section wise)
-
1) If the mass of the Sun were ten times smaller and the universal gravitational constant were ten times larger in magnitude, which of the following is not correct?
-
Time period of a simple pendulum on the Earth would decrease.
-
Walking on the ground would become more difficult.
-
Raindrops will fall faster.
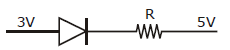
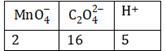
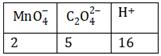
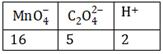
-
'g' on the Earth will not change.
-
-
2) A solid sphere is rotating freely about its symmetry axis in free space. The radius of the sphere is increased keeping its mass same. Which of the following physical quantities would remain constant for the sphere?
-
Rotational kinetic energy
-
Moment of inertia
-
Angular velocity
-
Angular momentum
-
-
3) A metallic rod of mass per unit length 0.5 kg m-1 is lying horizontally on a smooth inclined plane which makes an angle of 30° with the horizontal. The rod is not allowed to slide down by flowing a current through it when a magnetic field of induction 0.25 T is acting on it in the vertical direction. The current flowing in the rod to keep it stationary is
-
14.76 A
-
5.98 A
-
7.14 A
-
11.32 A
-
-
4) An inductor 20 mH, capacitor 100 μF and a resistor 50 Ω are connected in series across a source of emf, V = 10 sin 314 t. The power loss in the circuit is
-
2.74 W
-
0.43 W
-
0.79 W
-
1.13 W
-
-
5) A thin diamagnetic rod is placed vertically between the poles of an electromagnet. When the current in the electromagnet is switched on, the diamagnetic rod is pushed up, out of the horizontal magnetic field. Hence the rod gains gravitational potential energy. The work required to do this comes from
-
the lattice structure of the material of the rod
-
the magnetic field
-
the current source
-
the induced electric field due to the changing magnetic field
-
-
6) Current sensitivity of a moving coil galvanometer is 5 div/mA and its voltage sensitivity (angular deflection per unit voltage applied) is 20 div/V. The resistance of the galvanometer is
-
250Ω
-
25Ω
-
40Ω
-
500Ω
-
-
7) In the circuit shown in the figure, the input voltage Vi is 20 V, VBE = 0 and VCE = 0. The values of IB, IC and β are given by
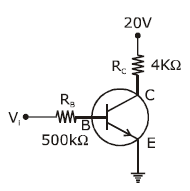
-
IB = 20μA, IC = 5mA, β = 250
-
IB = 25μA, IC = 5mA, β = 200
-
IB = 40μA, IC = 10mA, β = 250
-
IB = 40μA, IC = 5mA, β = 125
-
-
8) In a p-n junction diode, change in temperature due to heating
-
does not affect resistance of p-n junction
-
affects only forward resistance
-
affects only reverse resistance
-
affects the overall V – I characteristics of p-n junction.
-
-
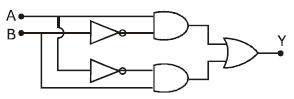
9) In the combination of the following gates the output Y can be written in terms of inputs A and B as
-
-
10) Suppose the charge of a proton and an electron differ slightly. One of them is –e, the other is (e+ ∆e). If the net of electrostatic force and gravitational force between two hydrogen atoms placed at a distance d (much greater than atomic size) apart is zero, then ∆e is of the order od [Given mass of hydrogen mh = 1.67 × 10-27 kg]
-
10-23 C
-
10-37 C
-
10-47 C
-
10-20 C
-
-
11) The resistance of a wire is ‘R’ ohm. If it is melted and stretched to ‘n’ times its original length, its new resistance will be:
-
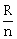
-
n2R
-
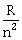
-
nR
-
-
12) The given electrical network is equivalent to:
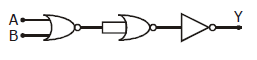
-
OR gate
-
NOR gate
-
NOT gate
-
AND gate
-
-
13) The de-Broglie wavelenght of a neutron in thermal equilibrium with heavy water at a temperature T (Kelvin) and mass m, is:
-
-
14) Which one of the following represents forward bias diode?
-
-
15) A long solenoid of diameter 0.1 m has 2 × 104 turns per meter. At the center of the solenoid, a coli of 100 turns and radius 0.01 m is placed with its axis coinciding with the solenoid axis. The current in the solenoid reduces at a constant rate of 0A form 4 A in 0.05 s. If the charge flowing through the coli during this time is:
-
16 μC
-
32 μC
-
16 πμC
-
32 πμC
-
-
16) Preeti reached the metro station and found that the escalator was not working. She walked up the stationary escalator in time t1. On other days, If she remains stationary on the moving escalator, then the escalator takes her up in time t2. The time taken by her to walk up on the moving escalator will be:
-
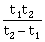
-
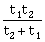
-
t1 – tz
-
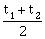
-
-
17) Young’s double slit experiment is first performed in air and then in a medium other than air. It is found that 8th bright fringe in the medium lies where 5th dark fringe lies in air. The refractive index of the medium is nearly.
-
1. 59
-
1. 69
-
1. 78
-
1. 25
-
-
18) A beam of light from a source L is incident normally on a plane mirror fixed at a certain distance x from the source. The beam is reflected back as a spot on a scale placed just above the source L. When the mirror is rotated through a small angle θ, the spot of the light is found to move through a distance y on the scale. The angle θ is given by:
-
-
19) Planck’s constant (h), speed of light in vacuum (c) and Newton’s gravitational constant (G) are three fundamental constant, which of the following combinations of these has the dimension of length?
-
-
20) Two cars P and Q start from a point at the same time a straight line and their positions are represented by xp (t) = at + bt2 and xQ (t) = ft – t2. At what time do the cars have the same velocity?
-
-
21) In the given figure, a = 15 m/s2 represents the total acceleration of a particle moving in the clockwise direction in a circle of radius R = 2.5 m at a given instant of time. The speed of the particle is
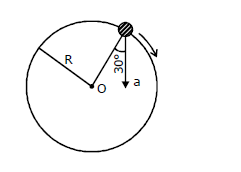
-
6.2 m/s
-
4.5 m/s
-
5.0 m/s
-
5.7 m/s
-
-
22) A rigid ball of mass m strikes a rigid all at 60° and gets reflected without loss of speed as shown in the figure below. The value of impulse imparted by the wall on the ball will be
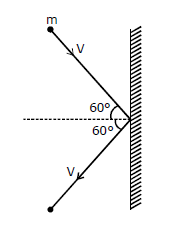
-
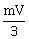
-
mV
-
2 mV
-
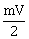
-
-
23) A bullet of mass 10 g moving horizontally with a velocity of 400 ms-1 strikes a wooden block of mass 2 kg which is suspended by a light inextensible string of length 5 m. As a result, the center of gravity of the block is found to rise a vertical distance of 10 cm. The speed of the bullet after it emerges out horizontally from the block will be
-
160 ms-1
-
100 ms-1
-
80 ms-1
-
120 ms-1
-
-
24) Two identically balls A and B having velocities of 0.5 m/s and -0.3 m/s respectively collide elastically in one dimension. The velocities of B and A after the collision respectively will be
-
0.3 m/s and 0.5 m/s
-
-0.5 m/s and 0.3 m/s
-
0.5 m/s and -0.3 m/s
-
-0.3 m/s and 0.5 m/s
-
-
25) A particle moves from a point
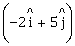 to
to 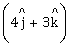 when a force of
when a force of 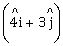 N is applied. How much work has been done by the force?
N is applied. How much work has been done by the force?-
2 J
-
8 J
-
11 j
-
5 J
-
-
26) Two rotating bodies A and B of masses m and 2 m with moments of inertia IA and IB (IB > IA) have equal kinetic energy of rotation. If LA and LB be be their angular momenta respectively, then
-
LA > LB
-
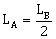
-
LA = 2LB
-
LB > LA
-
-
27) A solid sphere of mass m and radius R is rotating about its diameter. A solid cylinder of the same mass and same radius is also rotating about its geometrical axis with an angular speed twice that of the sphere the ratio of their kinetic energies of rotation (Esphere / Ecylinder) will be
-
3:1
-
2:3
-
1:5
-
1:4
-
-
28) A uniform rope of length L and mass m1 hangs vertically from a rigid support. A block of mass m2 is attached to the free end of the rope. A transverse pulse of wavelength λ1 is produced at the lower end of the rope. The wavelength of the pulse when it reaches the top of the ropes is λ2. The ratio λ2/ λ1 is:
-
-
29) A refrigerator works between 4°C and 30°C. It is required to remove 600 calories of heat every second in order to keep the temperature of the refrigerated space constant. The power required is: (Take 1 cal = 4.2 Joules)
-
2.365 W
-
23.65W
-
236.5W
-
2365W
-
-
30) An air column, closed at one end and open at the other, resonates with a tuning fork when the smallest length of the column is 50 cm, the next larger length of the column resonating with the same tuning fork is:
-
66.7 cm
-
100 cm
-
150 cm
-
200 cm
-
-
31) Consider the junction diode as ideal. The value of current flowing through AB is:

-
0 A
-
10-2 A
-
10-1 A
-
10-3 A
-
-
32) The charge flowing through a resistance R varies with time t as Q = at – bt2, where a and b are positive constants. The total heat produced in R is:
-
-
33) A black body is at a temperature of 5760 K. The energy of radiation emitted by the body at wavelength 250 nm is U1, at wavelength 500 nm is U2 and that at 1000 nm is U3. Wien’s constant, b = 2.88 × 106 nmK. Which of the following is correct?
-
U1 = 0
-
U3 = 0
-
U1 > U2
-
U2 > U1
-
-
34) Coefficient of linear expansion of brass and steel rods are α1 and α2. Length of brass and steel rods are l1 and l2 respectively. If (l2–l1) is maintained same at all temperatures, which one of the following relations holds good?
-
α1l2= α2l1
-
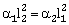
-
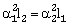
-
α1 l1 = α2 l2
-
-
35) A npn transistor is connected in common emitter configuration in a given amplifier. A load resistance of 800 Ω is connected in the collector circuit and the voltage drop across it is 0.8V. If the current amplification factor is 0.96 and the input resistance of the circuit is 192Ω, the voltage gain and the power gain of the amplifier will respectively be:
-
4, 3.84
-
3.69, 3.84
-
4, 4
-
4, 3.69
-
-
36) The intensity at the maximum in a Young’s double slit experiment is I0. Distance between two slits is d = 5λ, where λ is the wavelength of light used in the experiment. What will be the intensity in front of one of the slits on the screen placed at a distance D = 10 d?
-
-
37) 4.0 g of a gas occupies 22.4 litres at NTP, The specific heat capacity of the gas at constant volume is 5.0 JK-1. If the speed of sound in this gas at NTP is 952 ms-1, then the heat capacity at constant pressure is- (Take gas constant r = 8.3 JK-1 mo-1)
-
8.0 JK-1 mol-1
-
705 JK-1 mol-1
-
7.0 JK-1 mol-1
-
8.5 JK-1 mol-1
-
-
38) If vectors
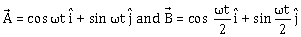 are functions of time, then the value of t at which they are orthogonal to each other is-
are functions of time, then the value of t at which they are orthogonal to each other is--
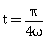
-
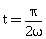
-
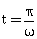
-
t = 0
-
-
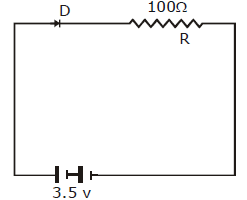
39) In the given figure, a diode D is connected to an external resistance R = 100 Ω and an e. m. f. of 3.5 V. If the barrier potential developed across the diode is 0.5 V, the current in the circuit will be-
-
30 mA
-
40 mA
-
20 mA
-
35 mA
-
-
40) If potential (in volts) in a region is expressed as V(x, y ,z) = 6xy – y + 2yz, the electric field (in N/C) at point (1, 1, 0) is-
-
-
41) A remote-sensing satellite of earth revolves in a circular orbit at a height of 0.25 × 106m above the surface of earth. If earth’s radius is 6.38 × 106m and g = 9.8 ms-1, then the orbital speed of the satellite is-
-
7.76 kms-1
-
8.56 kms-1
-
9.13 kms-1
-
6.67 kms-1
-
-
42) Two metal wires of identical dimensions are connected in series. If σ1 and σ2 are the conductivities of the metal wires respectively, the effective conductivity of the combination is
-
-
43) A satellite S is moving in an elliptical orbit around the earth. The mass of the satellite is very small compare to the satellite is very small compare to the mass of the earth. Then,
-
The angular momentum of S about the centre of the earth changes in direction, but its magnitude remains constant
-
The total mechanical energy of S varies periodically with time.
-
The linear momentum of S remains constant in magnitude
-
The acceleration of S is always directed towards the center of the earth
-
-
44) Two particles A and B, move with constant velocities
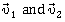 At the initial momentum their position vectors are
At the initial momentum their position vectors are 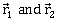 respectively. The condition for particles and B for their collision is-
respectively. The condition for particles and B for their collision is- -
-
45) Two stones of masses m and 2m are whirled in horizontal circles, the heavier one in a radius r/2 and the lighter one is radius r. The tangential speed of lighter stone is an times that of the value of heavier stone when they experience same centripetal forces. The value of n is-
-
2
-
3
-
4
-
1
-
-
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
-
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
-
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
-
1) Which one is a wrong statement?
-
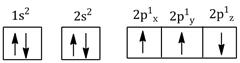
The electronic configuration of N atom is
-
An orbital is designated by three quantum numbers while an electron in an atom is designated by four quantum numbers.
-
Total orbital angular momentum of electron in ‘s’ orbital is equal to zero
-
The value of m for dZ2 is zero
-
-
2) Consider the following species:
CN+, CN- , NO and CN
Which one of these will have the highest bond order?
-
CN+
-
CN-
-
NO
-
CN
-
-
3) In the structure of CIF3, the number of lone pairs of electrons on central atom ‘CI’ is:
-
four
-
two
-
one
-
three
-
-
4) The correct order of atomic radii in group 13 elements is:
-
B < Ga < AI < TI < In
-
B < AI < Ga < In < TI
-
B < AI < In < Ga < TI
-
B < Ga < AI < In < TI
-
-
5) The correct order of N – compounds in its decreasing order of oxidation states is:
-
HNO3, NH4CI, NO, N2
-
HNO3, NO, NH4CI, N2
-
HNO3, NO, N2, NH4Cl
-
NH4CI, N2, NO, HNO3
-
-
6) Which one of the following elements is unable to from
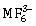 ion?
ion?-
B
-
Al
-
Ga
-
In
-
-
7) Which of the following statements is not true for halogens?
-
All shows positive oxidation states
-
All are oxidation agents
-
All from monobasic oxyacids
-
Chlorine has the highest electron-gain enthalpy
-
-
8) Considering Ellingham diagram, which of the following metals can be used to reduce alumina?
-
Mg
-
Zn
-
Fe
-
Cu
-
-
9) For the redox reaction
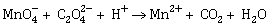
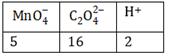
the correct coefficients of the reactants for the balanced equation are:
-
-
10) Ionic mobility of which of the following alkali metal ions is lowest when aqueous solution of their salts are put under an electric fields?
-
K
-
Rb
-
Li
-
Na
-
-
11) The most suitable method of separation of 1 : 1 mixture of ortho and para – nitrophenols is
-
Chromatography
-
Crystallisation
-
steam distillation
-
Sublimation
-
-
12) HgCl2 and I2 both when dissolved in water containing I- ions the pair of species formed is:
-
-
13) Mixture of chloroxylenol and terpineol acts as:
-
antiseptic
-
antipyretic
-
antibiotic
-
analgesic
-
-
14) An example of a sigma bonded organometallic compound is:
-
Grignard reagent
-
Ferrocene
-
Cobaltocene
-
Ruthenocene
-
-
15) A first order reaction has a specific reaction rate of 10-2 sec-1. How much time will it take for 20 g of the reactant of reduce to 5 g?
-
138.6 sec
-
346.5 sec
-
693.0 sec
-
238.6 sec
-
-
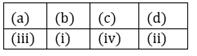
16) Match the interhalogen compound of column I with the geometry in column II and assign the correct code.
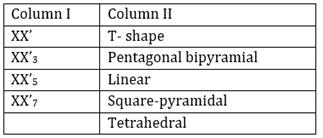
Codes:
-
-
17) Concentration of the Ag+ ions in a saturated solution of Ag2C2O4 is 2.2 × 10-4 mol L-1. Solubility product of Ag2C2O4 is:
-
2.66 × 10-12
-
4.5 × 10-11
-
5.3 × 10-12
-
2.42 × 10-8
-
-
18) In the electrochemical cell:
Zn|ZnSO4 (0.01 M) ∣∣ CuSO4 (1.0 M)| Cu, the emf of this Daniel cell is E1. When the concentration of ZnSO4 is changed to 1.0 M and that of CuSO4 is changed to 0.01 M, the emf changes to E2. From the followings, which one is the relationship between E1 and E2?
-
E1 < E2
-
E1 > E2
-
E2 = 0 ≠ E1
-
E1 = E2
-
-
19) The coagulation values in millimoles per litre of the electrolytes used for the coagulation of As2S3 are given below:
I. (NaCl) =52, II. (BaCl2) = 0.69, III. (MgSO4) = 0.22
-
III > I > II
-
I > II > III
-
II > I > III
-
III > II > I
-
-
20) During the electrolysis of molten sodium chloride, the time required to produce 0.10 mole of chlorine gas using a current of 3 amperes is
-
330 minutes
-
55 minutes
-
110 minutes
-
220 minutes
-
-
21) How many electrons can fit in the orbital for which n = 3 and 𝓁 = 1?
-
14
-
2
-
6
-
10
-
-
22) For a sample of perfect gas when its pressure is changed isothermally from pi to pf, the entropy change is given by
-
-
23) The van’t Hoff factor (i) for a dilute aqueous solution of the strong electrolyte barium hydroxide is
-
3
-
0
-
1
-
2
-
-
24) The percentage of pyridine (C5H5N) that forms pyridinium ion (C5H5N+H) in a 0.10 M aqueous pyridine solution (Kb for C5H5N = 1.7 × 10–9) is
-
1.6%
-
0.0060%
-
0.013%
-
0.77%
-
-
25) In calcium fluoride, having the fluorite structure, the coordination numbers for calcium ion (Ca2+) and fluoride ion (F–) are
-
4 and 8
-
4 and 2
-
6 and 6
-
8 and 4
-
-
26) If the E°cell for a given reaction has a negative value, which of the following gives the correct relationships for the values of ∆G° and Keq?
-
∆G° < 0; Keq < 1
-
∆G° > 0; Keq < 1
-
∆G° > 0; Keq > 1
-
∆G° < 0; Keq > 1
-
-
27) Which one of the following is incorrect for ideal solution?
-
∆Gmix = 0
-
∆Hmix = 0
-
∆Umix = 0
-
∆P = Pobs – Pcalculated by Rault's law
-
-
28) MY and NY3 two nearly insoluble salts, have the same Ksp value of 6.2 × 10-13 at room temperature. Which statements would be true in regard to MY and NY3?
-
The molar solubility of MY and NY3 in water are identical
-
The molar solubility of MY in water is less than that of NY3
-
The salts MY and NY3 are more soluble in 0.5 M KY than in pure water.
-
The addition of the salt of KY to solution of MY and NY3 will have no effect on their solubilities.
-
-
29) In a protein molecule various amino acids are linked together by:
-
α –glycosidic bond
-
β- glycosidic bond
-
peptide bond
-
dative bond
-
-
30) Natural rubber has:
-
All cis-configuration
-
All trans-configuration
-
Alternate cis-and trans-configuration
-
Random cis-and trans-configuration
-
-
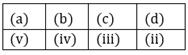
31) Match items of Column I with the items of Column II assign the correct code:
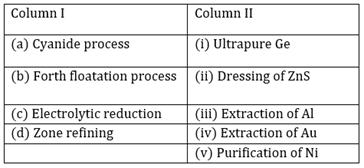
-
-
32) Which one of the following statements is correct when SO2 is passed through acidified K2Cr2O7 Solution?
-
The solution turns blue
-
The solution is decolourized
-
SO2 is reduced
-
Green Cr2 (SO4)3 is formed
-
-
33) The electronic configurations of Eu (Atomic No.63), Gd (Atomic No. 64) and Tb (Atomic No.65) are:
-
[Xe]4 𝑓7 6s2 , [Xe]4 𝑓8 6s2 and [Xe] 4 𝑓8 5d1 6s2
-
[Xe]4 𝑓6 5d1 6s2 , [Xe]4 𝑓7 5d1 6s2 and [Xe] 4 𝑓96s2
-
[Xe]4 𝑓6 5d1 6s2 , [Xe]4 𝑓7 5d1 6s2 and [Xe] 4 𝑓86s2
-
[Xe]4 𝑓76s2, [Xe]4 𝑓7 5d1 6s2 and [Xe] 4 𝑓96s2
-
-
34) Two electrons occupying the same orbital are distinguished by:
-
Principal quantum number
-
Magnetic quantum number
-
Azimuthal quantum number
-
Spin quantum number
-
-
35) When copper is heated with conc. HNO3 it produces:
-
Cu(NO3)2 and NO2
-
Cu(NO3)2 and NO
-
Cu(NO3)2,NO and NO2
-
Cu(NO3)2 and N2O
-
-
36) Which of the following reagents would distinguish cis-cyclopenta-1, 2-diol from the trans-isomer?
-
Acetone
-
Ozone
-
MnO2
-
Aluminium isopropoxide
-
-
37) A gas such as carbon monoxide would be most likely to obey the ideal gas law at:
-
Low temperatures and low pressures
-
High temperatures and low pressures
-
Low temperatures and high pressures
-
High temperatures and high pressures
-
-
38) If Avogardo number NA, is changed form 6.022 × 1023 mol−1 to 6.022 × 1020 mol−1, this would change:
-
The ratio of elements to each other in a compound.
-
The definition of mass in units of grams.
-
The mass of one mole carbon.
-
The ratio of chemical species of each other in a balanced equation.
-
-
39) Gadolinium belongs to 4f series. It’s atomic number is 64. Which of the following is the correct electronic configuration of gadolinium?
-
[Xe] 4f6 5d2 6s2
-
[Xe]4f8 5d2 6d2
-
[Xe]4f9 5s1
-
[Xe]4f7 5d1 6s2
-
-
40) What is the pH of the resulting solution when equal volumes of 0.1M NaOH and 0.01 M HCl are mixed?
-
1.04
-
12.65
-
2.0
-
7.0
-
-
41) Decreasing order of stability of
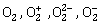
-
-
42) The correct statement regarding defects in crystalline solids is:
-
Frenkel defect is found in halides of alkaline metals.
-
Schottky defects have no effect the density of crystalline solids.
-
Frenkel defects decrease the density of crystalline solids.
-
Frenkel defect is a dislocation defect.
-
-
43) Which of the following statements is not correct for a nucleophile?
-
Nucleophiles are not electron seeking
-
Nucleophile is a Lewis acid
-
Ammonia is a nucleophile
-
Nucleophiles attack low e- density sites
-
-
44) The hybridization involved in complex [Ni(CN)4]2− is: (At. No. Ni = 28)
-
d2sp3
-
dsp2
-
sp3
-
d2sp2
-
-
45) The stability of +1 oxidation state among Al, Ga, In and Tl increase in the sequence:
-
In < Tl < Ga < Al
-
Ga < In < al < Tl
-
Al < Ga < In < Tl
-
Tl < In< Ga< Al
-
-
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
-
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
-
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
-
1) Casparian strips occur in
-
Cortex
-
Pericycle
-
Epidermis
-
Endodermis
-
-
2) Plants having little or no secondary growth are
-
Conifers
-
Deciduous angiosperms
-
Grasses
-
Cycads
-
-
3) A ‘new’ variety of rice was patented by a foreign company, though such varieties have been present in India for a long time. This is related to
-
Lerma Rojo
-
Sharbati Sonora
-
Co-667
-
Basmati
-
-
4) Which of the following is commonly used as a vector for introducing a DNA fragment in human lymphocytes?
-
λ – phage
-
Ti plasmid
-
Retrovirus
-
pBR 322
-
-
5) Use of bio resources by multinational companies and organisation without authorisation from the concerned country and its people is called
-
Bio-degradation
-
Bio-piracy
-
Bio-infringement
-
Bio-exploitation
-
-
6) Select the correct match:
-
T.H.Morgan - Transduction
-
F2 × Recessive parent - Dihybrid cross
-
Ribozyme - Nuclei acid
-
G. Mendel - Transformation
-
-
7) The correct order of steps in Polymerase Chain Reaction (PCR) is
-
Denaturation, Extension, Annealing
-
Annealing, Extension, Denaturation
-
Extension, Denaturation, Annealing
-
Denaturation, Annealing, Extension
-
-
8) In India, the organisation responsible for assessing the safety of introducing genetically modified organisms for public use is
-
Research Committee on Genetic Manipulation (RCGM)
-
Council for Scientific and industrial Research (CSIR)
-
Indian Council of Medical Research (ICMR)
-
Genetic Engineering Appraisal Committee (GEAC)
-
-
9) The stage during which separation of the paired homologous chromosomes begins is
-
Diakinesis
-
Diplotene
-
Pachytene
-
Zygotene
-
-
10) The Golgi complex participates in
-
Respiration bacteria
-
Formation of secretory vesicles
-
Fatty acid breakdown
-
Activation of amino acid
-
-
11) Stomatal movement is not affected by
-
O2 concentration
-
Light
-
Temperature
-
CO2 concentration
-
-
12) Stomata in grass leaf are
-
Rectangular
-
Kidney shaped
-
Dumb-bell-shaped
-
Barrel shaped
-
-
13) The two functional groups characteristic of sugars are
-
carbonyl and phosphate
-
carbonyl and methyl
-
hydroxyl and methyl
-
carbonyl and hydroxyl
-
-
14) Which of the following is not a product of light reaction of photosynthesis?
-
NADPH
-
NADH
-
ATP
-
Oxygen
-
-
15) Which of the following is true for nucleolus?
-
It takes part in spindle formation.
-
It is a membrane-bound structure.
-
Larger nucleoli are present in dividing cells.
-
It is a site for active ribosomal RNA synthesis.
-
-
16) Which among the following is not a prokaryote?
-
Nostoc
-
Mycobacterium
-
Saccharomyces
-
Oscillatoria
-
-
17) Winged pollen grains are present in
-
Mango
-
Cycas
-
Mustard
-
Pinus
-
-
18) After karyogamy followed by meiosis, spores are produced exogenously in
-
Agaricus
-
Alternaria
-
Neurospora
-
Saccharomyces
-
-
19) The vascular cambium normally gives rise to:
-
Primary phloem
-
Secondary xylem
-
Periderm
-
Phelloderm
-
-
20) Thalassemia and sickle cell anemia are caused due to a problem in globin molecule synthesis. Select the correct statement:
-
Both are due to quantitative defect in globin chain synthesis.
-
Thalassemia is due to less synthesis of globin molecules.
-
Sickle cell anemia is due to a quantitative problem of globin molecules.
-
Both are due to a qualitative defect in globin chain synthesis.
-
-
21) The genotypes of husband and wife are IAIB and IAi.
Among the blood types of their children, how many different genotypes and phenotypes are possible?
-
3 genotypes; 4 phenotypes
-
4 genotypes; 3 phenotypes
-
4 genotypes; 4 phenotypes
-
3 genotypes; 3 phenotypes
-
-
22) Which of the following facilitates the opening of stomatal aperture?
-
Decrease in turgidity of guard cells.
-
Radial orientation of cellulose microfibrils in the cell wall of guard cells.
-
Longitudinal orientation of cellulose microfibrils in the cell wall of guard cells.
-
Contraction of outer wall of guard cells.
-
-
23) In Bougainvillea thorns are a modification of:
-
Adventitious root
-
Stem
-
Leaf
-
Stipules
-
-
24) Which one of the following is related to ex-situ conservation of threatened animals and plants?
-
Biodiversity hot spots
-
Amazon rainforest
-
Himalayan region
-
Wildlife safari parks
-
-
25) Root hairs develop from the region of:
-
Elongation
-
Root cap
-
Meristematic activity
-
Maturation
-
-
26) A disease caused by an autosomal primary non-disjunction is:
-
Klinefelter's syndrome
-
Turner's syndrome
-
Sickle cell anemia
-
Down's syndrome
-
-
27) The water potential of pure water is:
-
Less than zero
-
More than zero but less than one
-
More than one
-
Zero
-
-
28) Which of the following options gives the correct sequence of events during mitosis?
-
Condensation → nuclear membrane disassembly → arrangement at equator → centromere division → segregation → telophase
-
Condensation → crossing over → nuclear membrane disassembly → segregation → telophase
-
Condensation → arrangement at equator → centromere division → segregation → telophase
-
Condensation → nuclear membrane disassembly → crossing over → segregation → telophase
-
-
29) The process of separation and purification of expressed protein before marketing is called
-
Downstream processing
-
Bioprocessing
-
Postproduction processing
-
Upstream processing
-
-
30) A temporary endocrine gland in the human body is:
-
Corpus cardiacum
-
Corpus luteum
-
Corpus allatum
-
Pineal gland
-
-
31) Which of the following is made up of dead cells?
-
Collenchyma
-
Phellem
-
Phloem
-
Xylem parenchyma
-
-
32) An example of colonial algae is:
-
Volvox
-
Ulothrix
-
Spirogyra
-
Chlorella
-
-
33) Match the following sexually transmitted diseases (Column-I) with their causative agent (Column-II) and select the correct option.

-
-
34) The function of copper ions in copper releasing IUDs is:
-
They inhibit gametogenesis.
-
They make the uterus unsuitable for implantation.
-
They inhibit ovulation.
-
They suppress sperm motility and fertilising capacity of sperms.
-
-
35) Which of the following in sewage treatment removes suspended solids?
-
Secondary treatment
-
Primary treatment
-
Sludge treatment
-
Tertiary treatment
-
-
36) An important characteristic that Hemichordates share with Chordates is:
-
Ventral tubular nerve cord
-
Pharynx with gill slits
-
Pharynx without gill slits
-
Absence of notochord
-
-
37) Radial symmetry is found in the flowers of
-
Cassia
-
Brassica
-
Trifolium
-
Pisum
-
-
38) Free-central placentation is found in
-
Citrus
-
Dianthus
-
Argemone
-
Brassica
-
-
39) Cortex is the region found between
-
Endodermis and vascular bundle
-
Epidermis and stele
-
Pericycle and endodermis
-
Endodermis and pith
-
-
40) The balloon-shaped structure called tyloses
-
Are linked to the ascent of sap through xylem vessels
-
Originate in the lumen of vessels
-
Characterise the sapwood
-
Are extensions of xylem parenchyma cells into vessels
-
-
41) A non-proteinaceous enzyme is
-
Deoxy ribonuclease
-
Lysozyme
-
Ribozyme
-
Ligase
-
-
42) Select the mismatch.
-
Methanogens - Prokaryotes
-
Gas vacuoles - Green bacteria
-
Large central vacuoles - Animal cells
-
Protists - Eukaryotes
-
-
43) Select the wrong statements.
-
Mycoplasma is a wall-less microorganism.
-
Bacteria cell wall is made up of peptidoglycan.
-
Pili and fimbriae are mainly involved in motility of bacteria cells.
-
Cyanobacteria lack flagellated cells.
-
-
44) A cell organelle containing hydrolytic enzymes is
-
Mesosome
-
Lysosome
-
Microsome
-
Ribosome
-
-
45) During cell growth, DNA synthesis takes place in
-
M phase
-
S phase
-
G1 phase
-
G2 phase
-
-
46) Which of the following biomolecules is common to respiration-mediated breakdown of fats, carbohydrates and proteins?
-
Acetyl CoA
-
Glucose-6-phosphate
-
Fructose 1, 6- bisphosphate
-
Pyruvic acid
-
-
47) A few drops of sap were collected by cutting across a plant stem by a suitable method. The sap was tested chemically. Which one of the following test results indicates that it is phloem sap?
-
Absence of sugar
-
Acidic
-
Alkaline
-
Low refractive index
-
-
48) You are given a tissue with its potential for differentiation in an artificial culture. Which of the following pairs of hormones would you add to the medium to secure shoots as well as roots?
-
Gibberellin and abscisic acid
-
IAA and gibberellin
-
Auxin and cytokinin
-
Auxin and abscisic acid
-
-
49) Phytochrome is a
-
Chromoprotein
-
Flavoprotein
-
Glycoprotein
-
Lipoprotein
-
-
50) Which is essential for the growth of root tip?
-
Mn
-
Zn
-
Fe
-
Ca
-
-
51) The process which makes major difference between C3 and C4 plants is
-
Respiration
-
Glycolysis
-
Calvin cycle
-
Photorespiration
-
-
52) Which one of the following statements is not correct?
-
Water hyacinth, growing in standing water, drains oxygen from water that leads to the death of fishes.
-
Offspring produced by asexual reproduction are called clones.
-
Microscopic, motile asexual reproductive structures are called zoospores.
-
In potato, banana and ginger, the plantlets arise from the internodes present in the modified stem.
-
-
53) Which one of the following generates new genetic combinations leading to variation?
-
Nucellar polyembryony
-
Vegetative reproduction
-
Parthenogenesis
-
Sexual reproduction
-
-
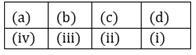
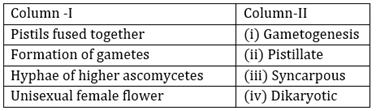
54) Match Column-I with Column II and select the correct option using the codes given below:
-
-
55) Microtubules are the constituents of:
-
Cilia, flagella and peroxisomes
-
Spindle fibres, centrioles and cilia
-
Centrioles, spindle fibres and chromatin
-
Centrosome, nucleosome and centrioles
-
-
56) A complex of ribosomes attached to a single strand of RNA is known as:
-
Polysome
-
Polymer
-
Polypeptide
-
Okazaki fragment
-
-
57) Fertilisation in humans is practically feasible only if:
-
The sperms are transported into vagina just after the release of ovum in fallopian tube.
-
The ovum and sperms are transported simultaneously to ampullary-isthmic junction of the fallopian tube.
-
The ovum and sperms are transported simultaneously to ampullary-isthmic junction of the cervix.
-
The sperms are transported into the cervix within 48 hrs of release of ovum in the uterus.
-
-
58) Asthma may be attributed to:
-
Bacterial infection of the lungs
-
Allergic reaction of the mast cells in the lungs
-
Inflammation of the trachea
-
Accumulation of fluid in the lungs
-
-
59) The Avena curvature is used for bioassay of:
-
ABD
-
GA3
-
IAA
-
Ethylene
-
-
60) The standard petal of a papilionaceous corolla is also called
-
Carina
-
Pappus
-
Vexillum
-
Corona
-
-
61) Tricarpellary, syncarpous gynoecium is found in flowers of:
-
Liliaceae
-
Solanaceae
-
Fabaceae
-
Poaceae
-
-
62) One of the major component of cell wall of most fungi is:
-
Chitin
-
Peptidoglycan
-
Cellulose
-
Hemicellulose
-
-
63) Select the incorrect statement:
-
FSH stimulates the Sertoli cells which help in spermiogenesis.
-
LH triggers ovulation in ovary.
-
LH and FSH decrease gradually during the follicular phase.
-
LH triggers secretion of androgens from the Leydig cells.
-
-
64) In meiosis crossing over is initiated at
-
Pachytene
-
Leptotene
-
Zygotene
-
Diplotene
-
-
65) A tall true breeding garden pea plant is crossed with a dwarf true breeding garden pea plant. When the F1 plants were selfed, the resulting genotypes were in the ratio of:
-
1 : 2 : 1 :: Tall homozygous : Tall heterozygous : Dwarf
-
1 : 2 : 1 :: Tall heterozygous : Tall homozygous: Dwarf
-
3 : 1 :: Tall : Dwarf
-
3 : 1 :: Dwarf : Tall
-
-
66) Which of the following is the most important cause of animals and plants being driven to extinction?
-
Over-exploitation
-
Alien species invasion
-
Habitat loss and fragmentation
-
Co-extinction
-
-
67) Which one of the following is a characteristic feature of cropland ecosystem?
-
Absence of soil organisms
-
Least genetic diversity
-
Absence of weeds
-
Ecological succession
-
-
68) Changes in GnRH pulse frequency in females is controlled by circulating level of:
-
Estrogen and progesterone
-
Estrogen and inhibin
-
Progesterone only
-
Progesterone and inhibin
-
-
69) Which of the following is not a feature of plasmids?
-
Independent replication
-
Circular structure
-
Transferable
-
Single-stranded
-
-
70) Which of the following features is not present in Periplaneta americana?
-
Schizocoelom as body cavity
-
Indeterminate and radial cleavage during embryonic development
-
Exoskeleton composed of N-acetylglucosamine
-
Metamerically segmented body
-
-
71) In higher vertebrates, the immune system can distinguish self-cells and non-self cells. If this property is lost due to genetic abnormality and it attacks self-cells, then it leads to:
-
Allergic response
-
Graft rejection
-
Auto-immune disease
-
Active immunity
-
-
72) Match the terms in Column-I with their description in Column-II and choose the correct option:
-
-
73) Golden rice is a genetically modified crop plant where the incorporated gene i's meant for biosynthesis of:
-
Vitamin B
-
Vitamin C
-
Omega 3
-
Vitamin A
-
-
74) Outbreeding is an important strategy of animal husbandry because it:
-
Helps in accumulation of superior genes.
-
Is useful in producing purelines of animals.
-
Is useful in overcoming inbreeding depression.
-
Exposes harmful recessive genes that are eliminated by selection.
-
-
75) Which one of the following hormones though synthesised elsewhere, is stored and released by the master gland?
-
Antidiuretic hormone
-
Luteinising hormone
-
Prolactin
-
Melanocyte stimulating hormone
-
-
76) An association of individuals of different species living in the same habitat and having functional interaction is:
-
Ecological niche
-
Biotic community
-
Ecosystem
-
Population
-
-
77) In which of the following both pairs have correct combination?
-
-
78) Identify the correct order of organisation of genetic material from largest to smallest:
-
Chromosome, gene, genome, nucleotide
-
Genome, chromosome, nucleotide, gene
-
Genome, chromosome, gene, nucleotide
-
Chromosome, genome, nucleotide, gene
-
-
79) A jawless fish, which lays eggs in fresh water and whose ammocoetes larvae after metamorphosis return to the ocean is:
-
Eptatretus
-
Myxine
-
Neomyxine
-
Petromyzon
-
-
80) Industrial melanism is an example of:
-
Neo Darwinism
-
Natural selection
-
Mutation
-
Neo Lamarckism
-
-
81) Cell wall is absent in:
-
Aspergillus
-
Funaria
-
Mycoplasma
-
Nostoc
-
-
82) The chitinous exoskeleton of arthropods is formed by the polymerisation of:
-
Keratin sulphate and chondroitin sulphate
-
D-glucosamine
-
N-acetyl glucosamine
-
Lipoglycans
-
-
83) Filiform apparatus is a characteristic feature of:
-
Generative cell
-
Nucellar embryo
-
Aleurone cell
-
Synergids
-
-
84) In angiosperms, microsporogenesis and megasporogenesis:
-
Occur in anther
-
Form gametes without further divisions
-
Involve meiosis
-
Occur in ovule
-
-
85) Metagenesis refers to:
-
Presence of different morphic forms
-
Alternation of generation between asexual and sexual phases of an organism
-
Occurrence of a drastic change in form during post-embryonic development
-
Presence of a segmented body and parthenogenetic mode of reproduction
-
-
86) Which of the following immunoglobulins does constitute the largest percentage in human milk?
-
lgD
-
lgM
-
lgA
-
lgG
-
-
87) Destruction of the anterior horn cells of the spinal cord would result in loss of:
-
Sensory impulses
-
Voluntary motor impulses
-
Commissural impulses
-
Integrating impulses
-
-
88) The cutting of DNA at specific locations became possible with the discovery of:
-
Restriction enzymes
-
Probes
-
Selectable markers
-
Ligases
-
-
89) In the following human pedigree, the filled symbols represent the affected individuals.
Identify the type of given pedigree.
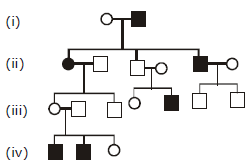
-
Autosomal dominant
-
X-linked recessive
-
Autosomal recessive
-
X-linked dominant
-
-
90) A colour blind man marries a woman with normal sight who has no history of colour blindness in her family. What is the probability of their grandson being colour blind?
-
0.5
-
1
-
Nil
-
0.25
-
-
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
-
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
-
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
-
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
-
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
-
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- 83
- 84
- 85
- 86
- 87
- 88
- 89
- 90