Class 10 NCERT Solutions Chemistry Chapter 2 - Acids, Bases and Salts
Acids, Bases and Salts Exercise 18
Solution 1
Step 2: A drop of the solution in test tube A is put on the red litmus paper. Same is repeated with solutions B and C. If either of them changes colour to blue, then it is basic. Therefore, out of three, one is eliminated.
Step 3: Out of the remaining two, any one can be acidic or neutral. Now a drop of basic solution is mixed with a drop of each of the remaining two solutions separately and then a drop of each solution is put on the red litmus paper.
If the colour of red litmus turns blue, then that solution is neutral and if there is no change in colour, then that solution is acidic.
This is because acidic and basic solutions neutralise each other. Hence, we can distinguish between the three types of solutions.
Concept insight: Remember that if the colour of red litmus paper gets changed to blue, then it is a base and if there is no colour change, then it is either acidic or neutral. Thus, basic solution can be easily identified. When acid and a base react with each other, they neutralise each other to form salt and water.
Acids, Bases and Salts Exercise 22
Solution 1
Curd and other sour substances contain acids. Therefore, when they are kept in brass and copper vessels, metals react with the acids to form harmful toxic products, thereby spoiling the food and damaging our health.
Concept insight: Remember that curd and sour substances contain acids in them. Acids react with metals to form salts and liberate hydrogen gas.Solution 2
Example:
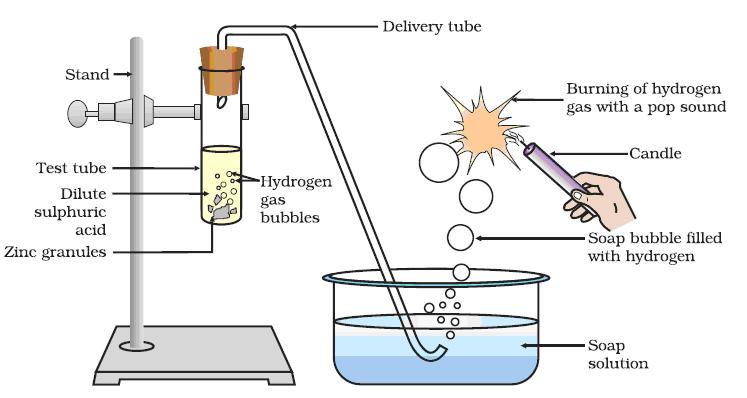
Procedure:
Step 1: Take few pieces of zinc granules and add 5 ml of dilute H2SO4.
Step 2: Shake it and pass the gas produced into a soap solution. Bubbles are formed in the soap solution. These soap bubbles contain hydrogen gas.
Zn + dil. H2SO4
Test for hydrogen gas: The evolved hydrogen gas can be tested by bringing a burning candle near the soap bubbles. Hydrogen gas burns with a pop sound.
Concept insight: You should be very cautious when you answer questions which have multiple parts. Answer each part separately and check at the end so that no answer is left out.
Solution 3
Metal compound A should be a compound of Ca as a calcium salt is formed in the product. We know that CO2 extinguishes a burning candle. Also, since carbon dioxide is liberated, therefore, it must be a carbonate. Hence, it is calcium carbonate.
CaCO3 (S) + 2HCl (aq) ![]() CaCl2 (S) + CO2 (g) + H2O (l)
CaCl2 (S) + CO2 (g) + H2O (l)
Concept insight: You should remember the properties shown by carbon dioxide. It extinguishes fire. Calcium carbonate reacts with dilute hydrochloric acid to form calcium chloride, carbon dioxide and water.
Acids, Bases and Salts Exercise 25
Solution 1
HCl and HNO3 show acidic character because they dissociate in the presence of water to form hydrogen or hydronium ions.
Although aqueous solutions of glucose and alcohol contain hydrogen, these do not dissociate in water to form hydrogen or hydronium ions. Hence, they do not show acidic character.
Concept insight: Remember that acids are those substances which dissociate in aqueous solutions to form hydrogen or hydronium ions.Solution 2
Acids dissociate in aqueous solutions to form charged particles called 'ions'. These ions are responsible for conduction of electricity.
Concept insight: The key to this answer lies in as to what happens when an aqueous solution of an acid is prepared.Solution 3
Dry HCl gas does not change the colour of the dry litmus paper because it does not contain hydrogen or hydronium ions.
Concept insight: The key to this answer lies in when does the colour of litmus paper change: acids change the colour of litmus paper to red.
Solution 4
It is recommended that the acid should be added to water and not water to the acid because the process of dissolving an acid in water is exothermic. If water is added to acid, since large amount of acid is present, a large amount of heat is generated at once. Hence, the mixture can splash out and cause burns. But, if acid is added to water, then heat is evolved gradually and easily absorbed by the large amount of water.
Concept insight: For answering this question, you should remember that the reaction between an acid and water is exothermic reaction.Solution 5
Concept insight: While answering this question, you should recall that dilution means adding of water to the acid.
Solution 6
Concept insight: While answering this question, you should recall that a base contains hydroxyl ions. When excess of base is added to a solution of sodium hydroxide, the concentration of hydroxide ions will increase.
Acids, Bases and Salts Exercise 28
Solution 1
A pH value of less than 7 indicates an acidic solution, while greater than 7 indicates a basic solution. The pH of a solution is inversely proportional to its hydrogen ion concentration. Hence, the solution having lower pH will have more hydrogen ion concentration. Therefore, the solution with pH = 6 is acidic and has more hydrogen ion concentration than the solution of pH = 8 which is basic.
Concept insight: While answering this question, you should remember that when pH < 7, the solution is acidic, when pH > 7, it is basic and when pH = 7, it is a neutral solution. When a solution is acidic, it will have more hydrogen ions and when a solution is basic it will have more hydroxide ions.Solution 2
Concept insight: Remember that concentration of H+ ions is directly proportional to the acidity of a solution.
Solution 3
Yes, a basic solution also has H+(aq) ions. These come from the ionization of water in which the base is dissolved. However, their concentration is less as compared to the concentration of OH-(aq) ions that makes the solution basic.
Concept insight: Remember that for a solution to be basic, the concentration of OH- ions should be more in a solution.Solution 4
If the soil is too acidic and improper for cultivation, then to neutralise the acidity of the soil, the farmer would treat the soil with bases like quick lime or slaked lime or chalk.
Concept insight: Remember that calcium oxide, calcium hydroxide and calcium carbonate are basic in nature. So, when an acid a base react, a neutralisation reaction takes place.Acids, Bases and Salts Exercise 33
Solution 1
Concept insight: For answering this question, you need to remember the formula of bleaching powder.
Solution 2
Calcium hydroxide or slaked lime [Ca(OH)2], on treatment with chlorine, yields bleaching powder.
Concept insight: For answering this question, you need to remember that which substance reacts with chlorine to give bleaching powder.Solution 3
Concept insight: For answering this question, you need to remember the uses of washing soda.
Solution 4
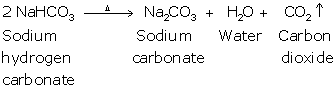
Solution 5
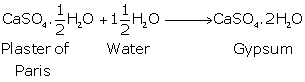
Concept insight: While answering this question, recall the formula of Plaster of Paris and what will happen when it will react with water.
Acids, Bases and Salts Exercise 34
Solution 1
Concept insight: Bases turn red litmus blue and acids turn blue litmus red. Basic solution has a pH value more than 7. Since the solution turns red litmus blue, its pH is likely to be 10.
Solution 2
Concept insight: Crushed egg shells contain calcium carbonate. Calcium carbonate reacts with HCl to liberate CO2 gas which turns lime water milky.
Solution 3
Solution 4
Solution 5
H2S04 (aq) + Zn(s)
(b) Hydrochloric acid + Magnesium
2HCl (aq) + Mg (s)
(c) Sulphuric acid + Aluminium
3 H2SO4 (aq) + 2 Al(s)
(d) Hydrochloric acid + Iron ![]() Ferric chloride + Hydrogen
Ferric chloride + Hydrogen
6 HCl (aq) + 2 Fe (s) ![]() 2 FeCl3 (aq) + 3H2 (g)
2 FeCl3 (aq) + 3H2 (g)
Concept insight: First convert the word equation into skeletal equation and then balance the different atoms on both the sides of the equation. Also, do not forget to write the symbols for different states.
Solution 6
Procedure:
Step 1: Two nails are fitted on a cork and are kept it in a 100 mL beaker.
Step 2: The nails are then connected to the two terminals of a 6-volt battery through a bulb and a switch.
Step 3: Some dilute HCl is poured in the beaker and the current is switched on.
Step 4: The same experiment is then performed with glucose solution and alcohol solution.
Observations: It will be observed that the bulb glows in the HCl solution and does not glow in glucose and alcohol solution.
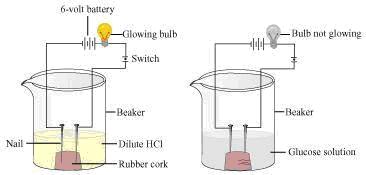
HCl dissociates into H+ (aq) and Cl- (aq) ions. These ions conduct electricity in the solution which results in the glowing of the bulb. On the other hand, glucose and alcohol solution do not dissociate into ions. Therefore, they do not conduct electricity.
Conclusion:
From this activity, it can be concluded that all acids contain hydrogen but not all compounds containing hydrogen are acids.
That is why, though alcohols and glucose contain hydrogen, they are not categorised as acids.
Concept insight: You should always mention the complete acitivity with complete procedure with diagram, observations, result in a sequence.
Solution 7
Distilled water is a pure form of water and is devoid of any ionic species. Therefore, it does not conduct electricity. When rain water falls to the earth, it dissolves an acidic gas 'carbon dioxide' from the air and forms carbonic acid. This acid provides some hydrogen ions and carbonate ions to rain water. Hence, due to presence of these ionic species, rain water conducts electricity.
Concept insight: For answering this question, you should always remember that ions are charge carriers. If there are no free ions avalaible, electricity will not be conducted.
Acids, Bases and Salts Exercise 35
Solution 8
Concept insight: For answering this question, you should always remember that acidic nature is because of the presence of H+ or H3O+ ions.
Solution 9
(b) Strongly alkaline- Solution C with pH 11
(c) Strongly acidic- Solution B with pH 1
(d) Weakly acidic- Solution A with pH 4
(e) Weakly alkaline- Solution E with pH 9
Concept insight: While answering this question, you should remember that when pH < 7, the solution is acidic, when pH > 7, it is basic and when pH = 7, it is a neutral solution. The lower the pH more will be the acidic nature. The higher the pH more will be the basic nature.
Solution 10
The fizzing will occur strongly in test tube A, in which hydrochloric acid (HCl) is added. This is because HCl is a stronger acid than CH3COOH and contains a much greater amount of hydrogen ions; therefore it produces hydrogen gas at a faster speed due to which fizzing occurs.
Concept insight: The key to this answer lies in the fact that HCl is a strong acd while CH3COOH is a weak acid.Solution 11
The pH of milk is 6. As it changes to curd, the pH will reduce because curd is acidic in nature due to formation of lactic acid.
Concept insight: Always remember that acids have pH < 7 and bases have pH >7.Solution 12
(a) The milkman shifts the pH of the fresh milk from 6 to slightly alkaline because in alkaline condition, milk does not get sour easily.
(b) Since this milk is slightly basic than usual milk, lactic acid produced to set the curd is neutralised by the base. Therefore, it takes a longer time for the curd to set.
Concept insight: Always remember that acids have pH < 7 and bases have pH >7.
Solution 13
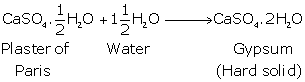
Concept insight: While answering this question, recall the formula of Plaster of Paris and what will happen when it will react with water.
Solution 14
For example:
(i) NaOH (base) + HCl (acid)
(ii) During indigestion (caused due to the production of excess of hydrochloric acid in the stomach), we administer an antacid (generally milk of magnesia, Mg(OH)2 which is basic in nature). The antacid neutralises the excess of acid produced and thus gives relief from indigestion.
Mg(OH)2 + 2 HCl
Concept insight: Always remember that the reaction of an acid and base to give salt and water is a neutralisation reaction.
Solution 15
Two important uses of washing soda and baking soda are as follows:
(1) Washing soda:
(a) It is used in glass, soap, and paper industries.
(b) It is used to remove permanent hardness of water.
(2) Baking soda:
(a) It is used as baking powder. Baking powder is a mixture of baking soda and a mild acid known as tartaric acid. When it is heated or mixed in water, it releases CO2 gas that makes bread or cake fluffy.
(b) It is used in soda-acid fire extinguishers.

