Class 9 MAHARASHTRA STATE TEXTBOOK BUREAU Solutions Science Chapter 4 - Measurement of Matter
Measurement of Matter Exercise Ex. 4
Solution 1
a. Positive Radicals : Na+, Fe2+,Ag+ Al3+.
b. Basic Radical : Na+, Fe2+,Al3+.
c. Composite Radical : SO4 2- ,NH4+
d. Metal With Variable Valency : Cu(1+,2+), Fe(2+,3+).
e. Bivalent Acidic Radical : S2- ,O2-
f. Trivalent Basic Radical : Al3+ , Fe3+.
Solution 2
|
Element |
Symbols |
Free radicals |
Charge of radicals |
|
1. Mercury |
Hg |
Mercurous- Hg+ |
+1 |
|
2. Potassium |
K |
Potassium- K+ |
+1 |
|
3. Nitrogen |
N |
Azide- N3- Nitrate- NO3- Nitrite-NO2- |
-1 |
|
4. Copper |
Cu |
Cuprous- Cu+ |
+1 |
|
5. Sulphur |
S |
Sulphide- S2- Sulphate- SO42- Sulphite- SO32- |
-2 |
|
6. Carbon |
C |
Carbide-C- ,triple carbene(:CH2) |
-1 |
|
7. Chlorine |
Cl |
Chloride- Cl- |
-1 |
|
8. Oxygen |
O |
Oxide- O2- |
-2 |
Solution 3
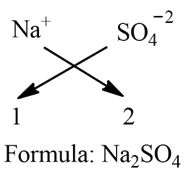
a) Sodium sulphate:
Step 1: Write the symbol of a basic radical (element with positive valency) to the left-hand side and that of the acid radical (element with negative valency) to the right-hand side.
Na SO4
Step 2: Write the valency of each of the respective radicals.
Na SO4
1 2
Step 3: Divide the valency by their highest common factor (HCF), if any, to get the simple ratio. Ignore (+) or (-) symbols of the radicals.
Step 4: Cross the reduced valencies. If 1 appears, then ignore it. If a group of atoms receives a valency more than 1, then enclose it within brackets.
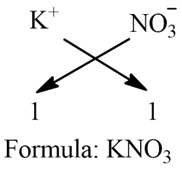
b) Potassium nitrate:
Step 1: Write the symbol of a basic radical (element with positive valency) to the left-hand side and that of the acid radical (element with negative valency) to the right-hand side.
K NO3
Step 2: Write the valency of each of the respective radicals.
K NO3
1 1
Step 3: Cross the reduced valencies. If 1 appears, then ignore it.
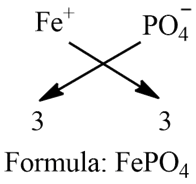
c) Ferric Phosphate:
Step 1: Write the symbol of a basic radical (element with positive valency) to the left-hand side and that of the acid radical (element with negative valency) to the right-hand side.
Fe PO4
Step 2: Write the valency of each of the respective radicals.
Fe PO4
3 3
Step 3: Divide the valency by their highest common factor (HCF), if any, to get the simple ratio. Ignore (+) or (-) symbols of the radicals.
Step 4: If a group of atoms receives a valency more than 1, then enclose it within brackets.
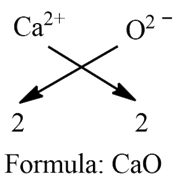
d) Calcium Oxide:
Step 1: Write the symbol of a basic radical (element with positive valency) to the left-hand side and that of the acid radical (element with negative valency) to the right-hand side.
Ca O
Step 2: Write the valency of each of the respective radicals.
Ca O
2 2
Step 3: Divide the valency by their highest common factor (HCF), if any, to get the simple ratio. Ignore (+) or (-) symbols of the radicals.
Step 4: If a group of atoms receives a valency more than 1, then enclose it within brackets.
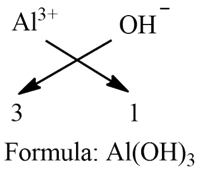
e) Aluminium Hydroxide:
Step 1: Write the symbol of a basic radical (element with positive valency) to the left-hand side and that of the acid radical (element with negative valency) to the right-hand side.
Al OH
Step 2: Write the valency of each of the respective radicals.
Ca O
2 2
Step 3: Divide the valency by their highest common factor (HCF), if any, to get the simple ratio. Ignore (+) or (-) symbols of the radicals.
Step 4: If a group of atoms receives a valency more than 1, then enclose it within brackets.
Solution 4
a) The valency of an element depends upon the number of electrons in its outermost shell. Since sodium has only one electron in its outermost shell its easy to lose it to complete its octet. Therefore its valency is 1 hence called as monovalent.
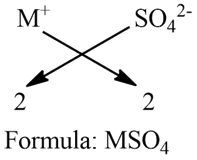
b) As given, M is a bivalent metal as M2+
Step 1: Write the symbol
M SO4
Step 2: Write the valency of each of the respective radicals.
M SO4
2 2
Step 3: If a group of atoms receives a valency more than 1, then enclose it within brackets.
Step 1: Write the symbol
M PO4
Step 2: Write the valency of each of the respective radicals.
M PO4
2 3
Step 3: If a group of atoms receives a valency more than 1, then enclose it within brackets.

c) The atomic mass of an element is the average relative mass of its atoms as compared with an atom of carbon-12.
· Earlier, hydrogen was taken as a standard for measuring atomic masses. Scientists compared the mass of an atom with the mass of hydrogen to determine its relative atomic mass.
· Hydrogen, being the smallest atom, its mass was taken as 1 unit. Example: If one atom of nitrogen weighs as much as 14 atoms of hydrogen, then the atomic mass of nitrogen is 14 units. · Later, carbon-12 isotope was chosen as a standard for measuring the atomic masses.
· In this system, carbon-12 is assigned a mass of exactly 12 atomic mass unit (amu) and masses of all other atoms are given relative to this standard. Recently, the unit of atomic mass amu was replaced by u, meaning unified mass.
d) The unified atomic mass unit (u), or Dalton (Da) or, sometimes, universal mass unit, is a unit of mass used to express atomic and molecular masses. It is the approximate mass of a hydrogen atom, a proton, or a neutron.
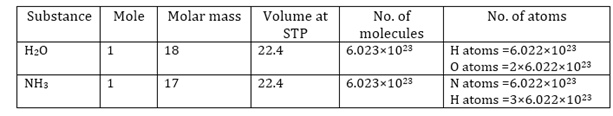
e) Mole: 1 mole of a substance is equal to its atomic mass or molecular mass expressed in grams.
The atomic mass expressed in grams is the gram atomic mass.
The molecular mass expressed in grams is the gram molecular mass.
For example
The atomic mass of sodium is 23 grams.
Therefore, 23 grams of sodium is equal to one mole of sodium atoms.
Similarly, the molecular mass of oxygen (O2) = 2 × Atomic mass of oxygen
= 2 × 16 = 32 g
So, 32 grams of oxygen is equal to one mole of oxygen molecules.
1 mole (of anything) = 6.022 × 1023 in number
Solution 5
a. Na2SO4 - Sodium sulphate
Molecular mass= sum of atomic masses of the elements present in a molecule
Molecular mass= 2 (23) + 32 + 4 (16)= 142g
b. K2CO3 - Potassium carbonate
Molecular mass= 2 (39) + 12 + 3 (16) = 138g
c. CO2 - Carbon dioxide
Molecular mass= 12 + 2 (16) = 44g
d. MgCl2 - Magnesium chloride
Molecular mass= 24 + 2 (35.5) = 95g
e. NaOH - Sodium hydroxide
Molecular mass= 23 + 16 + 1 = 40g
f. AlPO4 - Aluminium phosphate
Molecular mass= 27 + 31 + 4 (16) = 122g
g. NaHCO3 - Sodium bicarbonate Molecular mass= 23 + 1 + 12 + 3 (16) = 84g
Solution 6
As it is mentioned already:
Sample m Mass :7g
Mass of constituent oxygen :2g
Mass of constituent calcium :5g
Sample n Mass :1.4g
Mass of constituent oxygen :0.4g
Mass of constituent calcium:1.0g
Out of all the laws of chemical combination, this is proved by "Law Of Constant Proportion".
Law Of Constant Proportion states that "The proportion by weight of the constituent elements in various samples of compound is fixed in ratio".
for e.g: In sample m, the ratio of proportion of elements (calcium:oxygen) is 5:2
Ca:O =5:2
for e.g: In sample n, the ratio of proportion of elements (calcium:oxygen) is 1.0:0.4
Ca:O =1.0:0.4
=10:4
=5:2
On simplifying the ratio proportion by mass, we get the same values which verifies "The Law of Constant Proportion".
Solution 7
32g oxygen:
![]()
1 mole of O2 contains 6.022×1023 molecules
90g water:
![]()
5 moles of H2O contains = 5×6.022×1023 molecules
=30.11×1023 molecules
8.8g carbon dioxide:
![]()
0.2 moles of CO2 contains = 0.2×6.022×1023 molecules
=1.2044×1023 molecules
7.1g chlorine
![]() :
:
0.1 moles of chlorine contains = 0.1×6.022×1023 molecules
= 0.6022 ×1023 molecules
Solution 8
The formula :
Weight of substance (gm) = Number of moles × Molar Mass.
(i) For sodium chloride (NaCl):-
Weight of sodium chloride = Number of moles × molar mass.
= 0.2 × 58.44
= 11.69 grams.
(ii) For Magnesium Oxide (MgO):
Weight of magnesium oxide = Number of moles × Molar mass.
= 0.2 × 40.3
= 8.06 grams.
(iii) For calcium carbonate (CaCO3):
Weight of calcium carbonate = Number of moles × Molar Mass.
= 0.2 × 100.087
= 20.02 grams.

