Class 9 MAHARASHTRA STATE TEXTBOOK BUREAU Solutions Science Chapter 13 - Carbon : An important element
Carbon : An important element Exercise Exercise
Solution 1
a. A carbon atom forms a covalent bond with other atoms. In this bond the two atoms share electrons.
b. All the carbon bonds in a saturated hydrocarbon share electrons.
c. At least one carbon bond in an unsaturated hydrocarbon is double.
d. Carbon is the essential element in all the organic compounds.
e. The element hydrogen is present in all organic compound.
Solution 2
a. Most of the carbon compounds give a lot of heat and light when burnt in air. Saturated hydrocarbons burn with a clean flame and no smoke is produced. The carbon compounds, used as a fuel, have high calorific values. Therefore, carbon and its compounds are used as fuels for most applications.
b. Occurrence of carbon in nature in the solid state can be given as carbon is found in free state that means without combing with any other element in the form of diamond, graphite etc. Diamond, graphite contains elemental carbon only and not ant other element is included in the structure. But, carbon occurs in the combined state that means bonded with some other element other than carbon such as oxygen, calcium in the form of carbonates.
c. Uses of Diamond:
1) Diamond is used in jewellery as a well cut and polished diamond sparkles brightly.
2) Diamond is used to cut glass and other metals being the hardest substance. Diamond-tipped tools for cutting and drilling of rocks.
3) Diamond absorbs harmful radiation.
Solution 3
a. Diamond and graphite.
b. Crystalline and non-crystalline forms of carbon.
Solution 4
a. Graphite has delocalised Π electrons which are relatively free to move under the influence of electric field. Therefore graphite is a good conductor of electricity.
b. Graphite is not used in making ornaments because it is soft, brittle and slippery. It cannot be moulded like gold and silver and neither does it poses and lusture which is a desired characteristics in jewellery.
c. When CO2 is passed through lime water for a short duration, it turns lime water milky due to the formation of a white precipitate of calcium carbonate.
Ca(OH) + CO2 → CaCO3 + H2O
d. It was found that biogas has an effective role in climate change mitigation by reducing greenhouse gases emission. Biogas is a fuel with a high calorific value. It does not produce any smoke on burning. It can be easily ignited. It undergoes complete combustion. It can be directly supplied through pipes to our homes. It is a cheap fuel and generates manure as a byproduct.
Solution 5
a. Diamond, graphite and fullerenes are crystalline forms of carbon with a well-defined structure. The existence of a substance (an element) in two or more different forms (as of crystals) usually in the same phase is called allotropy. Crystalline forms of carbon have definite and regular geometry and have a long range as well as short range order of constituent particles.
b. Methane is called marsh gas because methane is found in marshy land. It is found in marshy land because when organism decomposes they produce methane.
c. Coal and petroleum are called fossil fuels because they are formed from the preserved remains of organisms that lived millions of years ago and are used as fuels.
d. Uses of Carbon:
· Diamond is a precious stone and is used in jewellery.
· Graphite being a good conductor is used for electrodes in batteries and in industrial electrolysis.
· Activated charcoal being highly porous is used in adsorbing poisonous gases and in water filters to remove organic contaminators.
· Carbon black is used as a black pigment in black ink and as a filler in automobile tyres.
· Coke is used as a fuel and largely as a reducing agent in metallurgy.
e. CO2 gas is used to extinguish the fire because it traps the flame and breaks down the supply of oxygen to the flame which is important for the combustion.
f. Uses of carbon dioxide:
(i) Carbon dioxide is used during photosynthesis in green plants.
(ii) It is used in preparation of chemicals like baking soda and washing soda.
(iii) It is used in the manufacturing of aerated drinks
(iv) It is used in fire extinguishers.
Solution 6
a. Physical Properties of Diamond:
1. Brilliant and pure diamond is the hardest natural substance.
2. The density of diamond is 3.5 g/cm3.
b. Physical Properties of Charcoal:
1. Graphite found in nature is black, soft, brittle and slippery.
2. The density of graphite is 1.9 to 2.3 g/cm3.
c. Physical Properties of Fullerene:
1. Molecules of fullerenes are found in the form of buckyballs and buckytubes.
2. There are 30 to 900 carbon atoms in one molecule of a fullerene.
3. Fullerenes are soluble in organic solvents such as carbon disulphide, chlorobenzene.
Solution 7
1. CH4 + 2O2→ CO2 + 2H2O + heat
2. CH4 + Cl2→ CH3Cl + HCl
3. 2NaOH + CO2→ Na2CO3 + H2O
Solution 8
a. Types of coal:
Anthracite: Highest and hardest quality coal.
Bituminous: It is Known as cooking coal as it is used to produce coke, coal gas and steam coal.
Lignite: Known as Brown coal. It is a lower grade coal.
Peat: It is the first stage of transformation of wood into coal.
Uses of coal
1. Coal is used in various industries as both domestic and an industrial fuel, that is, in homes, thermal power stations and furnaces.
2. It is processed in the industry to obtain useful products such as coke, coal tar and coal gas.
3. It is used in the manufacture of synthetic petrol.
4. It is also an important requirement for the manufacture of some fertilizers, drugs, rubber, wax, synthetic textiles and perfumes.
b.
Steps to prove that graphite conducts electricity:
Step 1: Take a sharpened pencil from both sides.
Step 2: Now, join a small bulb in the setup and connect it with a battery cell and connect its two ends to the both ends of pencil.
Step 3: Assemble the apparatus properly as shown in thee figure below and start the electric current in the circuit.
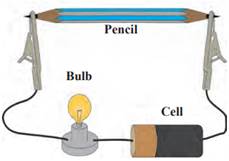
Observations: The bulb starts to glow as soon as the current is applied which proves that graphite is good conductor of electricity.
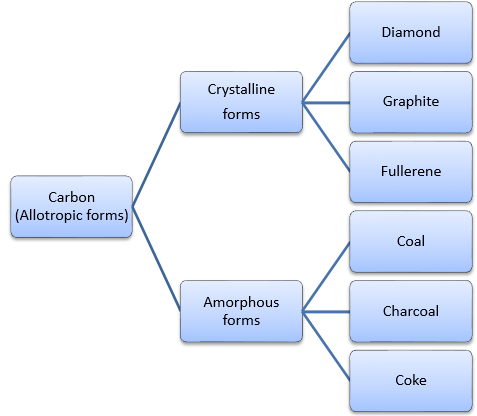
c. Allotropy: The existence of a substance (an element) in two or more different forms (as of crystals) usually in the same phase is called allotropy.
Allotropes of carbon
A. Crystalline forms: Diamond, graphite and fullerenes are crystalline forms of carbon with a well-defined structure. The existence of a substance (an element) in two or more different forms (as of crystals) usually in the same phase is called allotropy. Crystalline forms of carbon have definite and regular geometry and have a long range as well as short range order of constituent particles.
B. Amorphous forms: Coke, coal, charcoal, bone charcoal, etc. are non-crystalline allotropes of carbon. These are commonly called amorphous carbon.
d.
Solution 9
- Carbon dioxide is a colourless, odourless gas which is produced in the respiration process by animals and during combustion.
- It also turns the lime water into the milky white colour which indicates that the gas which is passed through the lime water is carbon dioxide.
- It also does not support the combustion or fire.
- It is consumed by plants during photosynthesis process.
- Frozen carbon dioxide gas is known as dry ice, which sublimates if the temperature will be increased, whereas the ice cube does not sublimate.

