Class 9 MAHARASHTRA STATE TEXTBOOK BUREAU Solutions Science Chapter 5 - Acids, Bases and Salts
Acids, Bases and Salts Exercise Ex. 5
Solution 1
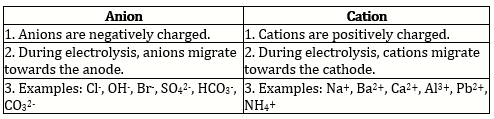
(a) Ammonium - Ammonium is cation and rest are anions.
(b) Hydrogen chloride - Hydrogen chloride is acid and rest are base.
(c) Acetic acid - Acetic acid is organic acid, others are inorganic acid
(d) Ammonium chloride - It is acidic salt and rest all are neutral salts.
(e) Sodium carbonate - Solution of sodium carbonate is basic, rest are neutral salt.
(f) Zinc oxide - It is amphoteric in nature and other ions are basic in nature
(g) Crystalline Common salt - Common salt don't have water of crystalisation. Like other salts.
(h) Acetic acid - acetic acid is weak electrolytes and rest are strong electrolytes.
Solution 2
(a) When 50 mL water is added to 50 mL solution of copper sulphate, then reversible reaction occurs and the colour change from pale blue to white. Also the concentration of copper sulphate solution decreases.
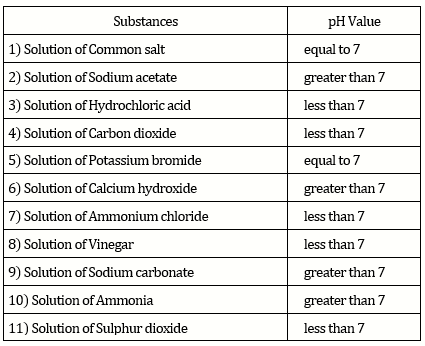
(b) The pH of neutral water solution is 7. And phenolphtalein shows pink colour on above pH 8 and NaOH has 13 hence it will sow pink colour on addition. Hence on adding water drops to phenolphthalein there is no change in colour but after addition of drops of NaOH it changes its colour from colourless to pink colour.
(c) Copper when reacts with dilute HNO3 forms Copper nitrate, Nitric oxide and water.
3Cu
+ 8 HNO3 ![]() 3Cu(NO3) 2 +4H2O
+ 2NO
3Cu(NO3) 2 +4H2O
+ 2NO
(d) When a red litums paper is dipped in an acidic solution, it does not have any effect on it. If it is base, then only it can turn it into blue colour.
(e) MgO does not react with NaOH. The reactions in which one reactant gets oxidized while the other gets reduced during the reaction are called oxidation-reduction reactions or redox reactions.
The reaction between manganese dioxide and hydrochloric acid is a redox reaction because MnO2 is reduced to MnCl2 and HCl is oxidized to H2O.
MnO2 + 4HCl → MnCl2 + Cl2 + 2H2O
Oxidizing agent - HCl Reducing agent - MnO2
(f) Zinc oxide is amphoteric because it reacts with both acids and bases to form salts.
ZnO + 2HCl → ZnCl2 + H2O
Zinc chloride
2NaOH + ZnO → Na2ZnO2 + H2O
Sodium zincate
(g) Dilute HCl on reaction with limestone or calcium carbonates liberates carbon dioxide gas, which is colourless, odourless gas which turns lime water milky.
![]()
(h) The copper sulphate crystals CuSO4.5H2O contain 5 water molecules which are known as water of crystallization. When we heat the crystals, these water molecules are removed and the salt turns white. If we moisten the crystals again with water, the blue colour of the crystals reappear.
![]()
Gypsum CaSO4.2H2O contains two water molecules as water of crystallization.
(i) During the process of electrolysis of water, dilute sulphuric acid splits into when electricity is passed through the dilute sulfuric acid solution. The electrolyte is dilute sulfuric acid which, during electrolysis is split into hydrogen and oxygen gases.
Solution 3
Oxides are of three types :
- Acidic Oxides: CO2 , SO3
- Basic Oxides: CaO, MgO, Na2O
- Amphoteric Oxides: ZnO, Al2O3 , Fe2O3
Solution 4
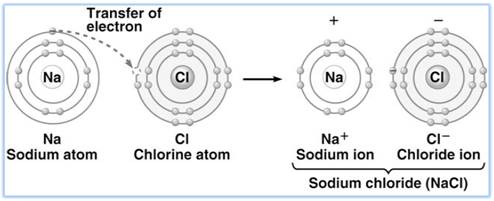
a. Formation of sodium chloride from sodium and chlorine.
The electronic configuration of sodium atom (At. No. 11) is 2, 8, 1. It readily loses an electron as it is highly electropositive to attain the stable configuration of the nearest noble gas atom and becomes a positively charged sodium cation (Na+ ).
![]()
Chlorine atom (electronic configuration: 2, 8, 7) requires one electron to acquire the stable electronic arrangement of an argon atom. So, it becomes a negatively charged chloride anion (Cl- ).
![]()
According to Kossel's theory, there is a transfer of one electron from the sodium atom to the chlorine atom and both atoms attain the noble gas configuration.
![]()
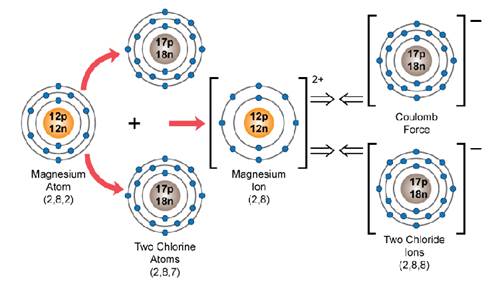
b. Formation of magnesium chloride from magnesium and chlorine:
The valency of magnesium is 2 and that of chlorine is 1. The magnesium atom will acquire a stable configuration of 8 electrons by losing 2 electrons from its outermost shell and become a positively charged magnesium ion, Mg2+ Mg - 2e-⎯⎯→ Mg 2+ (2,8,2) (2,8) However, chlorine will accept only 1 electron from the 2 electrons lost by the magnesium atom. Hence, these 2 electrons lost by the magnesium atom will be accepted by two chlorine atoms. 2Cl + 2e- ⎯⎯→ 2Cl- (2,8,7) (2,8,8) So, the ratio of magnesium to chloride ions in magnesium chloride is 1:2, so the molecular formula of the compound magnesium chloride is MgCl2. • Atomic or Orbit Structural Diagram
Solution 5
1. Hydrochloric acid : An aqueous solution of hydrochloric acid dissociates to form hydrogen ions. Since hydrogen ions do not exist as H+ in solution, they combine with polar water molecules to form hydronium ions [H3O+].
The proportion of dissociation is large.
HCl(aq) → H+(aq) + Cl- (aq)
2. Sodium chloride: Sodium
chloride is a salt which is an ionic compound. It is made up of sodium
(poitive ion) and chloride (negative ion). Water can dissolve salt because
the positive part of water molecules attracts the negative chloride and
negative part of water molecules attracts positive sodium. These positive and
negative ions are pulled apart by water molecules.
The proportion of dissociation is large.![]()
3. Potassium hydroxide: Potassium hydroxide is a base which dissolves in water to produce hydroxide ions. The proportion of dissociation is large.KOH(aq) → K+(aq) + OH- (aq)
4. Ammonia: It
dissolves in water to give ammonium hydroxide which ionizes to give hydroxyl
ions.
The proportion of dissociation is small.
NH3 + H2O ![]() NH4OH
NH4OH
NH4OH
NH4OH ![]() NH4++
OH-
NH4++
OH-
5. Acetic acid: Acetic acid is a monobasic acid which on ionization in water produce one hydronium ion per molecule of the acid. The proportion of dissociation is small. CH3COOH(aq) → H+(aq) + CH3COO- (aq)
6. Magnesium chloride: Magnesium chloride dissolves in water and forms magnesium ions and chloride ions. The proportion of dissociation is large. MgCl2(aq) → Mg+2(aq) + 2Cl- (aq)
7. Copper sulphate: Copper sulphate dissolves in water and forms copper ions and sulphate ions.
The proportion of dissociation is large. CuSO4 → Cu2+ + SO42-
Solution 6 (a)
7.3 g HCl in 100 mL solution :
Concentration of HCl in g L-1= =73 gL-1
In terms of gram per litre : 7.3 g of HCI in 100 mL has concentration = 73gL-1
Molecular mass of HCI = 1 + 35.5 = 36.5 Molarity= = 2mol L-1
In terms of moles per litre: 7.3 g of HCl in 100 mL has concentration = 2mol L-1
Solution 6 (b)
2 g NaOH in 50 mL solution : Concentration of NaOH in g L-1= ==40 gL-1
In terms of gram per litre : 2 g of NaOH in 50 mL has concentration = 40 gL-1
Molecular mass of NaOH = 23 + 16 + 1 = 40 Molarity= = 1mol L-1
In terms of moles per litre: 2 g of NaOH in 50 mL has concentration = 1mol L-1
Solution 6 (c)
3 g CH3COOH in 100ml solution :
Concentration of CH3COOH in g L-1= ==30 gL-1
In terms of gram per litre : 3 g of CH3COOH in 100 mL has concentration = 30 gL-1
Molecular mass of CH3COOH = 2×12+2×16+4×1=60 Molarity= = 0.5 mol L-1
In terms of moles per litre: 3 g of CH3COOH in 100 mL has concentration = 0.5mol L-1
Solution 6 (d)
4.9g H2SO4 in 200ml solution: Concentration of H2SO4 in g L-1= ==24.5 gL-1
In terms of gram per litre : 4.9g H2SO4 in 200ml solution has concentration = 24.5 gL-1
Molecular mass of H2SO4 = 2×12+2×16+4×1=60 Molarity= = 1mol L-1
In terms of moles per litre: 4.9g H2SO4 in 200ml solution has concentration
= 1mol L-1
Solution 7(a)
Depending on Basicity Basicity of an Acid The number of hydronium ions (H3O+) which can be produced by the ionisation of one molecule of that acid in aqueous solution. • Monobasic Acids Acids which on ionisation in water produce one hydronium ion (H3O+) per molecule of the acid are known as monobasic acids. Examples:
HCl + H2O ⇋ H3O+ + Cl- [Basicity = 1]
• Dibasic Acids Acids which on ionisation in water produce two hydronium ions (H3O+) per molecule of the acid are known as dibasic acids. Examples:
H2SO4 + H2O ⇋ H3O+ + HSO4- HSO4- + H2O ⇋H3O+ + SO4-2 [Basicity = 2]
Tribasic Acids Acids which on ionisation in water produce three hydronium ions (H3O+) per molecule of the acid are known as tribasic acids. Examples
H3PO4 + H2O ⇋ H3O+ + H2PO4- H2PO4-+ H2O ⇋ H3O+ + HPO4-2 HPO4-2⇋ H3O+ + PO4-3 [Basicity = 3]
Solution 7(b)
Neutralization is the process by which H+ ions of an acid react completely with the [OH]- ions of a base to give salt and water only.
![]()
Examples from everyday life of the neutralization reaction:
Indigestion
· Our stomach produces hydrochloric acid which helps us in the digestion process.
· But too much of acid in the stomach can cause indigestion which is painful.
· So, to relieve indigestion, an antacid such as milk of magnesia is taken, which contains magnesium hydroxide.
· Magnesium hydroxide neutralises the effect of excessive acid.
Ant Bite
· The sting of an ant contains formic acid.
· So when an ant bites, the effect of the acid can be neutralised by rubbing moist baking soda (sodium bicarbonate) or calamine solution which contains zinc carbonate.
Solution 7(c)
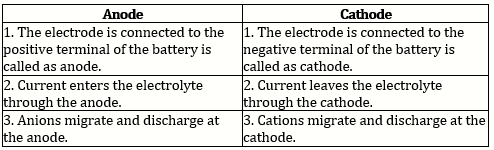
Electrolysis: It is the process of decomposition of a chemical compound in aqueous solutions or in molten state accompanied by a chemical change using direct electric current.
Electrolysis of water is the decomposition of water into hydrogen and oxygen on passage of electricity.
Electrodes:
Anode: Platinum
Cathode: Platinum
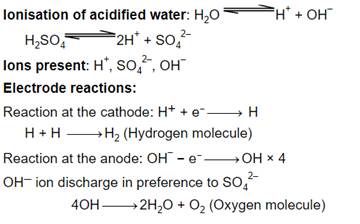
Ionisation of acidified water: H2O → H+ + OH-
H2SO4 → 2H+ + SO42-
Ions present: H+, SO42-, OH-
Electrode reactions:
Reaction at the cathode: H+ + e- → H
H + H → H2 (hydrogen molecule)
Reaction at the anode: OH- - e- → OH × 4
OH- ion discharge in preference to SO42-
4OH → 2H2O + O2 (oxygen molecule)
At the cathode, Hydrogen gas is evolved and at the anode, oxygen gas is liberated.
Solution 8
(a) NaOH + HCl → NaCl + H2O
(b) Zn +H2SO4 → ZnSO4+H2
(c) CaO + 2HNO3 → Ca(NO3)2 + H2O
(d) CO2 + KOH → H2O + K2CO3
(e) NaHCO3 + HCl → NaCl + H2O + CO2
Solution 9 (a)
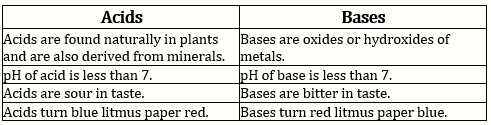
Solution 9 (b)
Solution 9 (c)