Class 8 MAHARASHTRA STATE TEXTBOOK BUREAU Solutions Science Chapter 7: Metals and Nonmetals
Metals and Nonmetals Exercise Ex. 7
Solution 1
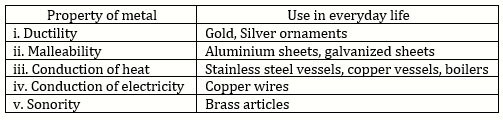
Solution 2
a. Diamond ( others are metals)
b. Brittleness ( other properties are metallic properties )
c. Bromine ( others are solid)
d. Iron ( others are alloys)
Solution 3
a.
(1) Stainless steel is an alloy of iron with carbon, chromium and nickel.
(2) The conductivity of copper is higher than that of iron in steel. Copper heats uniformly and faster. The time for cooking is reduced, as a result it saves fuel. Hence, the stainless steel vessels in kitchen have copper coating on the bottom.
b.
(1) Copper undergoes oxidation in air to form black copper oxide. Copper oxide reacts slowly with carbon dioxide in air and gains a green coat. This green substance is copper carbonate.
(2) Lemon contains acid. The acid dissolves the green coating of basic copper carbonate present on the surface of a tarnished copper and brass vessels and makes them shinyagain.
c.
(1) Sodium reacts so vigorously with atmospheric oxygen and water that it catches fire if kept in the open.
(2) It does not react with kerosene and sinks in it. Hence, to protect sodium and to prevent accidental fires it is always kept in kerosene.
Solution 4
a. By applying a layer of paint, oil, grease or varnish on the surface of a metal to prevent corrosion. Also plating with no corroding metal is done. Iron is coated with thin layer of zinc. Due to these processes the contact of metal surface with air is lost and corrosion in prevented.
b. The alloy brass is formed from copper and zinc and the alloy bronze is formed from copper and tin.
c. (1) A reddish coloured deposit (rust) is formed on iron by reaction with oxygen gas.
(2) A greenish coloured deposit (copper carbonate) is formed on copper by reaction with carbon dioxide.
(3) A blackish coloured deposit is formed (silver sulphide) on silver.
(4). Corrosion causes damages to carbides, bridges, iron railings, ships, especially those of iron, silver articles and copper vessels.
d. (1) Gold, silver and platinum are used to prepare ornaments.
(2) Silver is used in medicines. It has antibacterial property.
(3) Gold and silver are also used to make metals and few electronic devices.
(4) Platinum, palladium metals are used to catalyst.
Solution 5
- In the test tube 2, oil cuts the supply of air to nail due to which oxidation of nail is prevented and boiled water is free from gases. Hence, the nail in test tube 2 is not rusted.
- The nail in the test tube is highly rusted because nail is in contact with water and air. The oxidation process is fast.
- No change is observed in the test tube 3. Nail remains as it is because the calcium chloride absorbs moisture, making the air dry, thus preventing rusting of the nail.
