Class 8 MAHARASHTRA STATE TEXTBOOK BUREAU Solutions Science Chapter 6: Composition of Matter
Composition of Matter Exercise Exercise 6
Solution 1
a. Maximum
b. Incompressibility
c. Chemical composition of matter
d. Mixture
e. Heterogeneous mixture
f. Liquids
g. 2
Solution 2
a. Brass, It is an alloy while rest are elements.
b. Hydrogen, It is an element while the rest are compounds.
C. Carbon, It is element while rest are the mixture.
d. Petrol, as it is a mixture.
e. Sugar is organic compound while the rest are inorganic compounds.
f. Carbon, others are monovalent elements.
Solution 3
a. Photosynthesis :
![]()
Carbon dioxide, water, glucose and chlorophyll are compounds
Types :
1)Glucose : Organic Compound.
2)Chlorophyll : Complex Compound.
3)Carbon dioxide : Inorganic Compound.
4)Water : Compound.
b. Brass is an alloy, it contains 70% copper and 30% zinc. The largest proportion is solvent, i.e copper. The smaller proportion is solute, i.e zinc. The solution is brass.
c. (1) The constituents of a mixture (the proportions of salts in water) do not combine chemically.
(2) There constituents are present in any proportion by weight.
(3) The constituents of mixture can be separated by a physical process.
Solution 4
a. Mercury (Hg), Bromine (Br2)
b. Hydrogen (H2), Oxygen (O2)
c. Iron (Fe), Copper (Cu).
d. Sea water, Blue vitriol dissolved in water.
e. Milk, Blood.
f. Glucose , Urea
g. Chlorophyll, Haemoglobin.
h. Soda, Limestone.
i. Silicon, Arsenic.
j. Sodium (Na), Potassium (K)
k. Magnesium (Mg), Calcium (Ca).
Solution 5
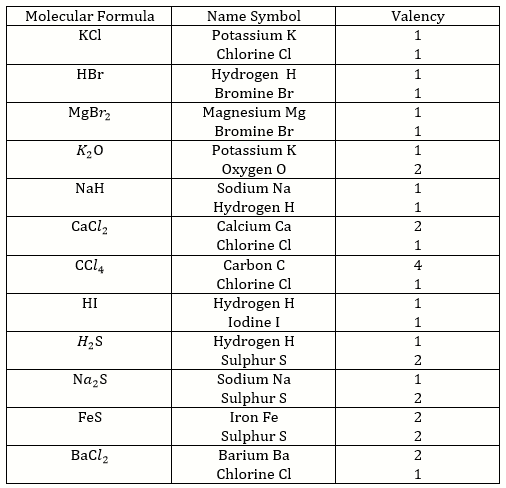
Solution 6
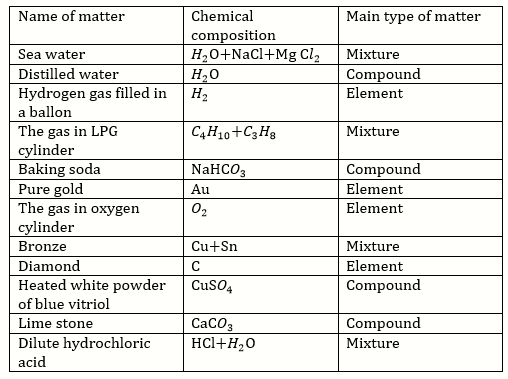
Solution 7
a. (1) Water is a compound of hydrogen and oxygen.
(2) In a compound, the constituents do not retain their individual properties. Hence hydrogen is combustible and oxygen helps in combustion, but water is neither combustible nor supports combustion, it helps to extinguish fire.
b. (1) A colloidal solution is heterogeneous.
(2) The diameters of colloidal particles are of the order of 10-5m. (3) The particles of a colloid can easily pass through a filter paper ,as the pore size of a filter paper is big. Hence, the constituents of colloidal cannot be separated by filtration.
c. (1) Lemon sherbat is a mixture. It is made up of lemon juice, sugar, salt and water.
(2) Formation of lemon sherbat doesnot involve any chemical reaction.
(3) The constituents of sherbat retain their individual properties. Hence, lemon sherbat is sweet, sour and salty to taste and it can be poured in a glass.
d. (1) The forces among the constituent particles (atom / molecule) are called inter molecular force.
(2) In solids, these forces are strong enough to keep the particles together in fixed positions, as a result solids have a definite shape and volume.
Solution 8
a. CCl4
b. NH3
c. C2O4
d. Ca2O2
