Class 10 MAHARASHTRA STATE TEXTBOOK BUREAU Solutions Science Chapter 5 - Heat
Heat Exercise Ex. 5
Solution 1
a. The amount of water vapour in air is determined in terms of its absolute humidity.
b. If objects of equal masses are given equal heat, their final temperature will be different. This is due to difference in their specific heat capacities.
c. During transformation of liquid phase to solid phase, the latent heat is released.
Solution 2
- Substances expand on heating and contract on cooling. However, water is an exception. Water contract on heating and expand on cooling within a specific range of temperature. This is called anomalous expansion.
- When water at 0 °C is heated, it is observed that it contracts between 0 °C and 4 °C. Beyond 4 °C, it expands normally. Similarly, when it is cooled from 4 °C to 0 °C, it expands.
- The expansion of water when it is cooled from 4 °C to 0 °C is known as the anomalous expansion of water.
- The volume of water is minimum at 4 °C.
Solution 3
The amount of heat energy required to raise the temperature of a unit mass of an object by 1 °C is called the specific heat capacity of that object.
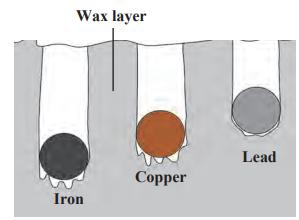
- Take three spheres of iron, copper and lead of equal mass as shown in figure above.
- Put all the three spheres in boiling water in the beaker for some time.
- Take the three spheres out of the water.
- All the spheres will be at temperature 100°C. Put them immediately on the thick slab of wax.
- Note, the depth that each of the sphere goes into the wax.
- The sphere which absorbs more heat from the water will give more heat to wax. More wax will thus melt and the sphere will go deeper in the wax. It can be observed that the iron sphere goes deepest into the wax. Lead sphere goes the least and copper sphere goes to intermediate depth. This shows that for equal rise in temperature, the three spheres have absorbed different amounts of heat. This means that the property which determines the amount of heat absorbed by a sphere is different for the three spheres. This property is called the specific heat capacity.
Solution 4
- While deciding the unit for heat, temperatures interval chosen is from 14.5°C to 15.5°C.
- The amount of heat necessary to raise temperature of 1 g of water by 1 °C from 14.5°C to 15.5°C is called one cal heat.
- Similarly, the amount of heat necessary to raise the temperature of 1 kg of water by 1°C from 14.5 °C to 15.5°C is called one kcal heat.
Solution 5
- The temperature of the mixture remains 0° C till the ice melts completely.
- When heating continues, even after conversion of all the ice into water, the temperature of water starts rises and reaches 100° C. At this temperature water starts converting into steam. The temperature of water remains constant at 100° C till all water converts into steam.
- In this graph, line AB represents conversion of ice into water at constant temperature.
- When ice is heated it melts at 0° C and converts into water at this constant temperature.
- The ice absorbs heat during this transition and the absorption of energy continues till all the ice gets converted into water.
- The temperature remains constant during this transition.
- This constant temperature, at which the ice converts into water is called the melting point of ice.
- Thus, during transition of solid phase to liquid, the object absorbs heat energy, but its temperature does not increase. This heat energy is utilised for weakening the bonds between the atoms or molecules in the solid and transform it into liquid phase.
- The heat energy absorbed at constant temperature during transformation of solid into liquid is called the latent heat of fusion.
Solution 6.a
The anomalous expansion of water helps preserve aquatic life during very cold weather. When temperature falls, the top layer of water in a reservoir contract, becomes denser and sinks to the bottom. A circulation is thus set up until the entire water in the pond reaches its maximum density at 4°C. If the temperature falls further, the top layer expands and remains on the top till it freezes. Thus, even though the upper layer is frozen the water near the bottom is at 4°C and thus aquatic life like fishes etc. can survive in it easily.
Solution 6.b
- The bottle taken out of refrigerator is at lower temperature as compared to the outer environment.
- It cools the air surrounding it.
- Air in the vicinity becomes saturated with vapour and the excess vapour condenses on the surface of water bottle in form of water droplets. This happens because air reaches its dew point temperature.
Solution 6.c
Water has the tendency to expand below 4°C. Thus, in cold regions when the temperature falls below 4°C, the water content present in rocks expands. Due to this expansion of water or increase in volume of water, the rocks cracks.
Solution 7.a
Latent heat of a body is the amount of heat required to change the state of unit mass of the body from solid to liquid or from liquid to gas without any change in temperature. If latent heat is given off, then the body in liquid state will transform to solid state and the body in vapour state will transform to liquid state.
Solution 7.b
Principle of heat exchange is used in calorimetry method to determine the specific heat capacity of a substance.
Solution 7.c
Latent heat is the quantity of heat absorbed or released by a substance undergoing a change of state, such as ice changing to water or water to steam, at constant temperature.
The latent heat of fusion of ice is the amount of heat energy required to change ice at 0°C into water at the same temperature.
The latent heat of vaporization of steam is the amount of heat energy required to change water at 100°C to steam at the same temperature.
Latent heat = Heat given or taken/ mass = Q/m
Solution 7.d
For a given volume of air, at a specific temperature, there is a limit on how much water vapour the air can contain. When the air contains maximum possible water vapour, it is said to be saturated with vapour at that temperature. The amount of vapour needed to saturate the air depends on temperature of the air. If air temperature is low, it will need less vapour to saturate the air. If the vapour contained in air is less that the maximum limit, then the air is said to be unsaturated.
Solution 8
- Heat is transferred from the object at higher temperature to object at lower temperature.
- Principle of heat exchange
- Heat energy lost by the hot object = Heat energy gained by the cold object. This is called as 'Principle of heat exchange'
- Specific heat of substance.
Solution 9.a
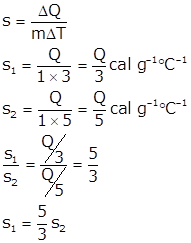
Specific heat of body A is more than B by 5/3
Solution 9.b
Amount of heat energy released in cooling 2 kg water from 20°C to 0°C = 2 x 1000 × 1 × 20 = 40000 cal
Amount of heat energy released in converting 2 kg water at 0°C to ice = 2 x 1000 × 80 = 160000 cal
Thus, total energy for converting water at 20°C to ice = 200000 cal
Grams of ammonia to be evaporated = 200000/341 = 586.5 g
Solution 9.c
Amount of heat required in converting 150 g ice to 0°C = 150 × 80 = 12000 cal
Amount of heat energy required in heating 150 g water at 0°C to 150 g water at 50°C = 150 ×1 × 50 = 7500 cal
Total heat energy required to convert 150 g ice at 0°C to water 50°C = 19500 cal
The amount of heat released in converting m g of steam at 100°C to water at 100°C = m × 540
Amount of heat released in converting m g of water at 100°C to water at 50°C = m × 1 × 50 = 50 m
Total energy released to convert m g steam at 100°C to water at 50°C = 590 m cal
By principle of calorimetry,
590 m = 19500
m = 19500/590 = 33 g
Solution 9.d
Let T be the final temperature of mixture.
Amount of heat required in converting 10 g of ice to 0 °C to water at 0 °C = 10 x 80 = 800 cal
Amount if heat required to convert 10 g water at 0 °C to water at T °C = 10 × 1 × T = 10 T
Total heat energy required to convert 10 g ice at 0 °C to water at T °C = 800 + 10 T
Amount of heat released to raise the temperature of calorimeter at 30°C to T °C = 100 × 0.1 × (30 - T) = 10 (30 - T)
Amount of heat released to raise temperature of 250 g of water at 30 °C to T °C = 250 × 0.4 × (30 - T)
Total amount of heat released in process = 110 (30 - T)
By principle of calorimetry,
110(30 - T) = 800 + 10 T
T = 20.83 °C

