Class 12-science H C VERMA Solutions Physics Chapter 5 - Specific Heat Capacities of Gases
Specific Heat Capacities of Gases Exercise 77
Solution 1
Mechanical energy is converted into internal energy. Thus,
![]()
![]()
![]()
Solution 2
We know,
dQ=dU+dW
dQ=dU (As dW=0)
![]()
![]()
Q=8.6cal
Or Q=36.12J
Solution 3
We know,
dW=PdV
![]()
![]()
dW=300J
Also, dW=nRdt
![]()
And ![]()
![]()
![]()
dQ=1050J
Solution 4
We know,
![]()
So
![]()
![]()
Also,
![]()
![]()
![]()
Solution 5
a) We know,
dQ=dU+dW
dU=dQ-dU
![]()
![]()
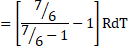
=6RdT
![]()
dU=2490J
b) dU=dQ
![]()
![]()
![]()
dU=2490J
c) Adiabatically ![]()
![]()
![]()
![]()
![]()
dU=2490J
Specific Heat Capacities of Gases Exercise 78
Solution 6
We know,
PV = nRT
![]()
![]()
![]()
Also,
![]()
![]()
![]()
Also,
![]()
![]()
=51.18
![]()
![]()
=2.08cal
Solution 7
a) We know,
dQ=dU+dW
dU=dQ-dW
=dQ-PdV
![]()
dU=30J
b) For monoatomic gas
![]()
![]()
n=0.008
c) We know,
![]()
![]()
![]()
And ![]()
=12.5+8.3=20.3
d) From equation 1
![]()
Solution 8
We know,
![]()
![]()
![]()
![]()
Also,
![]()
![]()
Q=3nRT
Or
![]()
![]()
Solution 9
As given:
P=kV
![]()
![]()
RdT=2kVdV
![]()
We know,
dQ=dU+dW
![]()
![]()
![]()
![]()
Solution 10
As given
![]()
![]()
![]()
![]()
![]()
![]()
Solution 11
For ![]() ideal gas:
ideal gas:
![]()
For ![]() ideal gas:
ideal gas:
![]()
As given ![]()
i.e. ![]()
![]()
When we mix the gas
![]()
![]()
![]()
![]()
![]()
Also,
![]()
Taking ratio of equation 1 and 2
![]()
Solution 12
We know,
![]()
![]()
![]()
![]()
Also,
![]()
![]()
Ratio ![]()
![]()
Solution 13
a) We know,
![]()
![]()
![]()
![]()
b) We know,
![]()
![]()
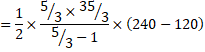
=1250J
Also, dQ=dU+dW [[For bc process, dW=0]
![]()
![]()
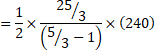
![]()
c) Heat liberated, ![]()
![]()
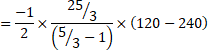
=750J
d) Also, Heat libeated,![]() (for cd process)
(for cd process)
![]()
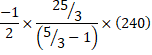
=2500J
Solution 14
a) In case of ab , ![]() is constant
is constant
Thus, ![]()
![]()
![]()
In case of bc, Pis constant
Thus ![]()
![]()
![]()
b) Work done=Area under graph
![]()
c) We know,
![]()
![]()
![]()
Q=14.9J
And also,
![]()
![]()
![]()
Q=24.9J
d) We know,
![]()
![]()
=(24.9+14.9)-1 [from1 and 2]
![]()
Solution 15
We know
![]()
![]()
![]()
Solution 16
a) We know
![]()
![]()
![]()
b) We know,
![]()
![]()
![]()
![]()
Solution 17
a) ![]()
![]()
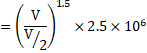
![]()
b)
![]()
![]()
![]()
![]()
c) Workdone is given as:
![]()
![]()
W=21J
Solution 18
A diabetic process
Thus,
![]()
![]()
![]()
![]()
![]()
Solution 19
a) We know
![]()
![]()
![]()
![]()
Also,
![]()
![]()
![]()
b) When the container is slowly compressed then also heat transferred is 0 because walls are adiabatic and thus
Pressure p=800kPa
And temperature T=600K
Solution 20
a) First slowly compressed ∴Isothermal compression.
So,
![]()
![]()
Now, suddenly compressed ∴Adiabatic compression.
![]()
![]()
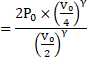
![]()
b) First gas is
compressed suddenly![]() Adiabatic compressin
Adiabatic compressin
![]()
![]()
![]()
![]()
Now, gas is compressed slowly ∴ Isothermal compression.
![]()
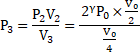
![]()
Solution 21
a) Isothermal condition
![]()
![]()
![]()
Now, adiabatic condition. Thus,
![]()
![]()
![]()
b) Adiabatic condition:
![]()
![]()
Now, isothermal condition,
![]()
![]()
Solution 22
a) We know,
PV=nRT
![]()
n=0.009moles
b) We know,
![]()
![]()
![]()
c) Adiabatic process ∴
![]()
![]()
![]()
Or
![]()
d) We know,
![]()
![]()
![]()
W=-33J
e) We know, internal energy
![]()
![]()
W=33J
Specific Heat Capacities of Gases Exercise 79
Solution 23
A is isothermal, Thus.
![]()
![]()
![]()
B is adiabatic. Thus,
![]()
![]()
![]()
C is isobaric process. Thus,
![]()
![]()
And ![]()
As given
![]()
![]()
Ratio is given as:
![]()
![]()
Solution 24
Work done is given as:
![]()
And![]()
As work done is equal for both cases, Thus,
![]()
![]()
Also we know,
![]()
![]()
Substituting above equation in 3 we get
![]()
![]()
Solution 25
a) Adiabatic process, Thus,
![]()
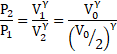
![]()
b) We know,
![]()
![]()
![]()
And also
![]()
![]()
![]()
Now
![]()
![]()
![]()
c) We know,
dQ=dU+dW
dU=-dW [As dQ=0]
dU=+82J
d) from equation 1
![]()
![]()
e) Isobaric process as pressure is constant. Thus,
![]()
![]()
f) Here, ![]()
![]()
![]()
For isothermal condition,
![]()
![]()
![]()
g) Net work done, ![]()
=-82-41.4+103
=-20.4J
Solution 26
In this, adiabatic process is going on. So
![]()
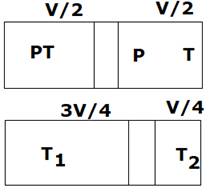
So, ![]()
![]()
Solution 27
a) We know,
PV=nRT
![]()
n=0.008
b) We know,
dQ=dU+dW
dQ=dU (dW=0,as no change in volume)
![]()
![]()
For A,
![]()
And ![]()
![]()
![]()
For B,
![]()
And ![]()
![]()
![]()
Distance moved by
mercury =![]()
=25-12.5
![]()
Solution 28
Adiabatic process, Thus.
For ![]() ,
, ![]()
![]()
For ![]() ,
, ![]()
![]()
Where m is mass of H2
Equating 1 and 2 we get,
![]()
m=0.029
m=0.03g
Solution 29
a) Temperature remains constant as A is diathermic
Thus, ![]()
![]()
![]()
And ![]()
Now as B is adiabatic,
![]()
![]()
Solving we get
![]()
Also,
![]()
![]()
![]()
![]()
b) Here, temperature remains constant because value is open.
![]()
And
![]()
A is diathermic thus, T and V are constant and so pressure also remains constant.
![]()
B is adiabatic. Thus,
![]()
![]()
Or ![]()
Solution 30
a) Adiabatic process,
![]()
And
![]()
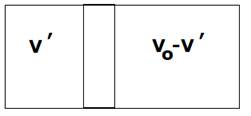
![]()
When equilibrium is reached then
![]()
![]()
Solving we get
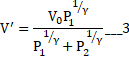
And
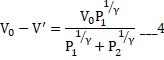
b) Process is adiabatic so no heat is transferred to left part.
Q=0
c) From equation 1
![]()
And from equation 3
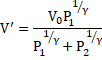
Thus, equation 5 becomes:
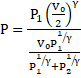
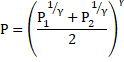
Solution 31
Process is adiabatic. Thus,
![]()
![]()
![]()
![]()
Also,
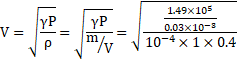
V=447m/s
Specific Heat Capacities of Gases Exercise 80
Solution 32
We know,
Spedd of sound, ![]()
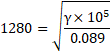
![]()
Also,
![]()
=18.0J/mol-x
Also,
![]()
![]()
=26.3J/mol-K
Solution 33
We know,
![]()
![]()
![]()
Also, speed od sound V=![]()
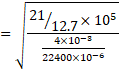
V=960m/s
Solution 34
In kundt's tube, node separation is given as:
![]()
![]()
Speed of sound ![]()
![]()
=360m/s
Also,
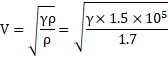
Thus,
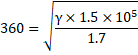
![]()
Now, we know,
![]()
![]()
Also,
![]()
![]()
![]()
Solution 35
Speed of sound ![]()
![]()
=330m/s
Also,
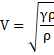
{PV=nRT
![]() }
}
![]()
So, ![]()
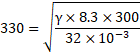
![]()
We know,
![]()
![]()
And
![]()
![]()
![]()
![]()

