Class 12-science H C VERMA Solutions Physics Chapter 21 - Bohr's Model and Physics of Atom
Bohr's Model and Physics of Atom Exercise 384
Solution 1
Dimensions of RHS=![]()
=![]()
=(![]()
Dimension of length
Solution 2
Wavelength emitted
![]()
![]() Quantum number of final state
Quantum number of final state
![]() Quantum number of initial state
Quantum number of initial state
a) n=3 to n=2
![]()
![]()
![]()
b) n=5 to n=4
![]()
![]()
c) n=10 to n=9
![]() (1.097
(1.097![]()
![]()
Solution 3
Smallest wavelength means energy should be maximum, so transition will be from infinity to the ground state.
![]()
Hence, ![]()
Hydrogen
![]() ]
]
![]()
He+
![]()
![]()
Li++
![]()
λ= 10 nm
Solution 4
Rydberg constant
![]()
=![]()
![]()
Solution 5
Energy of Hydrogen atom in n=2 state
![]()
=![]()
![]()
So, Binding Energy 3.4ev
Solution 6
Radius=0.529![]() ; Energy=
; Energy=![]() Ev
Ev
n=1
![]() ; Energy=
; Energy=![]()
![]() ; E
; E![]()
n=4
![]()
![]()
n=10
![]() ;
; ![]()
![]()
Solution 7
The radiation lies in the ultraviolet region so n1=1
![]()
![]()
![]()
Transition will be from n=3 to n=1
Solution 8
First excitation means transition from n=1
![]()
![]()
![]()
![]()
![]()
![]()
Ionisation Potential means transition from n=1 to n=![]()
![]()
![]()
![]()
Solution 9
Wavelength
![]()
Three Transitions are possible:
n=4 to n=2
![]()
![]()
n=4 to n=3
![]() =
=![]()
![]()
n=3 to n=2
![]()
![]()
Solution 10
Energy for photon of wavelength
![]()
![]()
The transition of electron by this photon is from n=2 to n=1
![]()
![]() ) eV
) eV
![]()
![]()
![]()
The ion may be Helium.
Solution 11
![]() [Minimum radius is for first orbit]
[Minimum radius is for first orbit]
![]()
![]() N
N
Solution 12
![]()
![]()
n=4
Now,
![]()
![]()
n=2
n=4 to n=2
![]()
![]()
![]()
Solution 13
From n=2 to n=1
![]()
![]() )
)
![]()
Solution 14
Energy released in transition from n=6 to n=1 =Energy released in 1st transition +Energy released in 2nd transition
=>![]()
![]()
![]()
![]()
![]()
![]()
In 2nd transition , electron transit from nth state to n=1
![]()
![]()
![]()
Solution 15
Potential Energy=2×total energy ; Kinetic Energy |Total Energy|
=2![]() =
=![]() eV
eV
For n=1;PE=![]()
PE=27.2eV
For n=2;PE=![]()
PE -6.8ev……………………………………………………KE=3.4ev
O make potential energy zero in ground state we have to add 27.2ev in all states.
So, for n=2, new potential energy=-6.8ev+27.2ev
PE'=20.4ev
K.E will remain same KE'=KE=3.4ev
Total energy=PE'=KE'
=20.4+3.4
TE'=23.8ev
Solution 16
![]()
![]()
For λ= 46nm transition is from n=3 to n=1
![]()
![]()
![]()
![]()
For λ=103.5, transition is from n=3 to n=2
![]()
![]()
![]()
![]()
Solution 17
No. of spectral lines![]()
![]()
![]()
n=4
Solution 18
![]()
![]()
![]()
For ![]() , n will be minimum , so n=1
, n will be minimum , so n=1
![]()
![]()
Solution 19
The range of wavelength falling in Balmer series is between 656.3nm and 365nm.
Number of wavelength in the range=![]()
= 36
Two lines will be extra for the first and last wavelength
∴ Total number of lines = 36+2
=38.
Solution 20
Smallest wavelength means maximum energy is emitted.
So transition will be from ![]()
![]()
![]()
![]() (b) Transition will be in balmer series as visible region from n=4 to n=2
(b) Transition will be in balmer series as visible region from n=4 to n=2
![]()
![]()
Solution 21
Frequency of revolution of electron ![]()
![]()
![]()
![]()
λ![]()
![]()
Bohr's Model and Physics of Atom Exercise 385
Solution 22
![]()
![]()
![]()
At such higher temperature, hydrogen get dissociated into atoms.
Solution 23
![]()
![]()
![]()
![]()
Solution 24
Time to complete 1 revolution by![]() in n=2 state
in n=2 state
![]()
Let N revolutions done in![]()
![]()
![]()
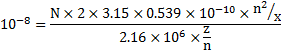
Put, n=2 and z=1
![]()
Solution 25
Dipole Moment
![]()
![]()
![]()
![]()
![]()
![]()
Solution 26
![]() )
)![]()
![]()
![]()
![]()
![]()
Solution 27
Given wavelength lies in the visible region so, e0 in n=2 may absored some wavelength andwill get excites.
![]()
![]()
![]()
So, λ=487 nm will be absorbed as it lies between 450 nm to 550 nm.![]()
Solution 28
Energy released by ![]()
![]()
![]()
![]()
This energy is used to excite electron of Helium ions from n=1 to nth state
![]()
![]()
![]()
![]()
No integer. Hence, not possible.
Solution 29
![]()
![]()
Binding energy of electron in ground state=![]()
![]()
![]()
![]()
![]()
![]()
Solution 30
![]()
![]()
![]()
Energy required for transition![]()
|
TRANSITION |
ENERGY REQUIRED |
ENERGY REMAININ G |
WAVELENGTH |
|
n=1 to n=2 |
|
|
|
|
n=1 to n=3 |
|
|
|
|
N=1 to n=4 |
= |
absorbed |
|
|
|
|
|
|
The electron is in n=2 andwill be excite from
n=3 to n=2
![]()
λ =654 nm
n=3 to n=1
![]()
λ =103 nm
n=2 to n=1
![]()
λ=121 nm
Solution 31
To ionize hydrogen atom, energy required=13.6 eV
For PEE,
![]()
=>13.6 ![]() =
=![]()
=>![]()
![]()
![]()
![]()
Energy required for n=1 to n=2 excitation
![]()
![]()
Now,
![]()
![]()
![]()
![]()
![]()
For visible light , it will be emission in Balmer series
For ![]() , E has to minimum so excitation will be from n=1 n=3
, E has to minimum so excitation will be from n=1 n=3
![]()
![]()
From PEE,
![]()
![]()
![]()
![]()
![]()
![]()
Solution 32
The emitted wavelength lies in visible region (Balmer series) so it will de-excite from nth state to n=2 state
![]()
![]()
n=3
So, Energy required to excite electron from n=1 to n=3
![]()
![]()
E=12.1 eV
Charge x potential=12.1 eV
e(Pot)=12.1 eV
Pot=12.1 volt
Electric field![]()
![]()
Solution 33
In elastic collision between two bodies of equal masses, the velocity gets interchange.
Since the hydrogen atom is at rest, after collision, the velocity of the neutron will be zero.
Solution 34

Momentum conservation(![]()
![]()
![]()
Energy loss![]()
![]()
![]()
![]()
Energy used for ionization of one atom of hydrogen
![]()
Here,
![]()
![]()
![]()
![]()
Solution 35
For minimum speed v, collision will be perfectly inelastic , the loss in Kinetic Energy is used to excite electron for n=1 to n=2
![]()
![]()
![]()
![]()
Solution 36
![]()
؞![]()
0=![]()
|![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() eV
eV
![]()
Solution 37
Difference in energy from n=3 to n=2
![]()
![]()
Recoil of atom
(.) Momentum conservation
![]()
![]()
![]()
![]()
(.) Energy conservation
![]()
![]()
Solution 38
Energy in![]() light=
light=![]()
![]()
=1.90 eV
Solution 39
Foe maximum work function , maximum energy of Balmer's series will be from n=∞![]() to n=2 transition
to n=2 transition
![]()
![]()
E=3.4eV
Solution 40
For maximum Kinetic Energy, Energy of photons should be maximum
So, transition will be from n=∞ to n=1
![]()
![]() =13.6 eV
=13.6 eV
![]()
KE =E - ∅
=13.6eV-1.9eV
KE=11.7eV
Solution 41
![]()
![]()
![]()
Solution 42
Gravitational force
![]()
![]()
By bohr's rule
![]()
![]()
![]()
Equating (i) and (ii)
![]()
For minimum radius
n=1
r=![]()
r=![]()
![]()
![]()
![]()
Bohr's Model and Physics of Atom Exercise 386
Solution 43
Gravitational force
![]()
![]()
By bar's rule
![]() (ii)
(ii)
From (i) and (ii)
![]()
![]()
![]()
![]()
![]()
Potential energy =![]()
![]() )
)
![]()
![]()
![]()
Solution 44
![]()
![]()
![]()
![]()
![]()
![]()
Radius
![]()
From (i)
![]()
![]()
![]()
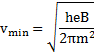
Solution 45
Longest wavelength , energy should be minimum and it will be for n=2 to n=4
![]()
![]()
E = 2.55 ev
![]()
![]()
λ = 487 nm.
Solution 46
Velocity of hydrogen atom in state 'n' =u
Also the velocity of photon=u
But u ≪ c
Here photon is emitted as a ware
So , its velocity is same as that of hydrogen atom i.e.; u
![]()
Frequency ![]()
![]()
![]()
![]()

