The anodic half-cell of lead-acid battery is recharged using electricity of 0.05 Faraday. The amountof PbSO4 electrolyzed in g during the process is : (Molar mass of PbSO4 = 303 g mol—1)
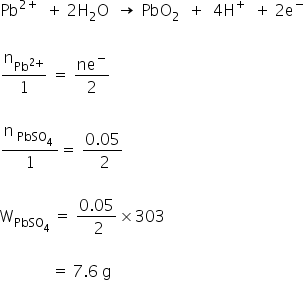
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
You have rated this answer 10/10
For Franchisee Enquiry
or
You are very important to us
For any content/service related issues please contact on this number
93219 24448 / 99871 78554
Mon to Sat - 10 AM to 7 PM
The anodic half-cell of lead-acid battery is recharged using electricity of 0.05 Faraday. The amountof PbSO4 electrolyzed in g during the process is : (Molar mass of PbSO4 = 303 g mol—1)
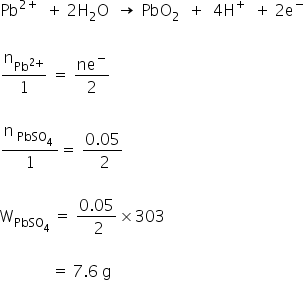
You have rated this answer 10/10