JEE Plances Class Answered
q.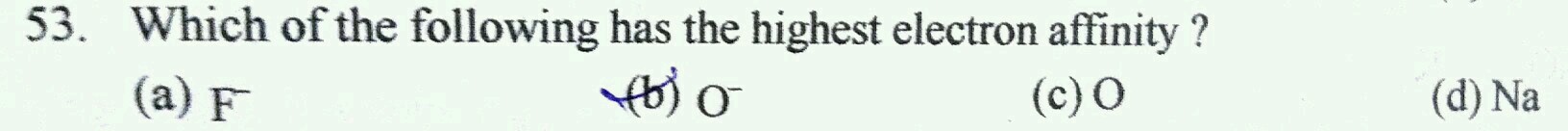
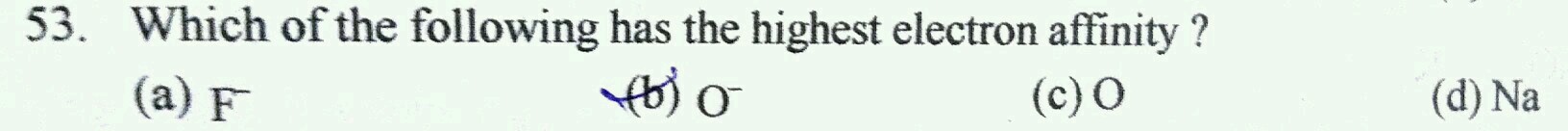
Asked by Arushi Juyal | 16 Dec, 2017, 07:27: AM
F- is already negatively charged and its octet is complete hence it is neutral, so eliminate that
Na is electropositive instead of accepting it will lose electrons, hence it is no incentive to add electrons, eliminate this too
Now between O and O-,
O has the highest affinity towards electrons than O-.
Hence O is the correct answer.
Answered by Ramandeep | 17 Dec, 2017, 11:26: AM

