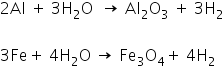ICSE Class 8 Answered
Reaction of Metals with Water:
Metals react with water and produce metal oxide with the release of hydrogen gas. However, all metals do not react with water.
Metal oxides which are soluble in water dissolve to form hydroxide.
Metal + Water → Metal oxide + Hydrogen
Metal oxide + Water → Metal hydroxide\ |
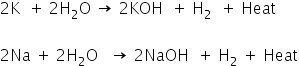
2. Magnesium does not react with cold water but reacts with hot water to form magnesium hydroxide with the evolution of hydrogen gas.
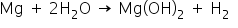
3. Metals such as aluminium, zinc, and iron do not react with cold or hot water, but they react with steam to form a metal oxide and hydrogen.