ICSE Class 8 Answered
Differentiate between saturated and supersaturated solution.
Asked by Chandrashekhar | 18 Sep, 2018, 09:29: PM
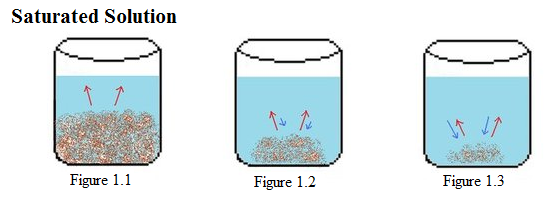
Solution in which no more solutes can be added to solution i.e the amount of solute = amount of solvent.
In other words -
A solution is said to be saturated when a solute is not able to dissolve in the solvent. As more and more solute is added to the solvent, it gets to a point where the solvent cannot dissolve any more solute because of some particular conditions (note, the ability of solute to dissolve various amounts of solvent varies and depends on the conditions like temperature, pressure etc). This is when the solution becomes saturated. If you add any more solute, it settles at the bottom of the container. For instance, when water is mixed with lemonade crystals, the lemonade crystals dissolve. At the point when no more lemonade crystals can dissolve in the water, the solution is said to be saturated.
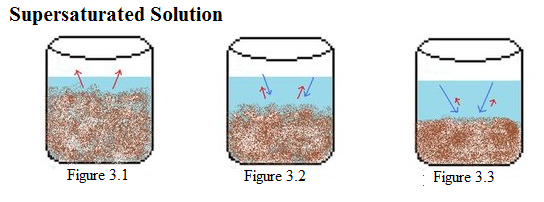
A supersaturated solution, on the other hand, is when the excess of solute is dissolved in the solvent as a result of changes in temperature, pressure or other conditions. At room temperature, a saturated solution keeps the maximum possible amount of solute, and the rest becomes excess. If any more solute is added to the solvent, it will not dissolve but rather settle at the bottom of the container. However, if temperatures are increased, the solvent can dissolve additional solute that was initially settled at the bottom. Even if the solution is then cooled down to room temperature, it still can hold this excess of solute dissolved for some time. This is exactly when it becomes supersaturated. But it’s a temporary condition; eventually the excess of solvent will precipitate and the solution will become saturated again.
Answered by Sumit Chakrapani | 19 Sep, 2018, 12:40: AM
Application Videos
Concept Videos
ICSE 8 - Chemistry
Asked by ppsk4364 | 20 Apr, 2024, 11:22: AM
ICSE 8 - Chemistry
Asked by poonamaashi24 | 19 Apr, 2024, 06:49: PM
ICSE 8 - Chemistry
Asked by vinodlucknow251 | 08 Mar, 2024, 02:27: PM
ICSE 8 - Chemistry
Asked by sivakrishnanarisetty | 07 Oct, 2023, 04:18: PM
ICSE 8 - Chemistry
Asked by saisuhas410 | 01 Oct, 2023, 05:00: PM
ICSE 8 - Chemistry
Asked by angelinemaria2010 | 22 Sep, 2023, 05:41: PM
ICSE 8 - Chemistry
Asked by n.tara345 | 16 Aug, 2023, 06:50: PM
ICSE 8 - Chemistry
Asked by kshyapi | 24 Jul, 2023, 12:12: AM
ICSE 8 - Chemistry
Asked by kumarashutosh1331 | 23 Jul, 2023, 07:44: AM
ICSE 8 - Chemistry
Asked by enakshipal07 | 21 Jul, 2023, 11:45: AM











