ICSE Class 9 Answered
Take a thin walled glass tube, about 15 cm long and in it slip a strip of mm graph paper, all along its length. Hold the strip with cellotape. Put enough lead shots in the tube, such that it floats upright in water. Measure the length of tube inside water. Let the length be l1 mm.
Now take a cylinder filled with alcohol and float the tube in it. Measure the length of tube inside alcohol. Let it be l2 mm.
Therefore, Density of alcohol = 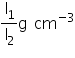
Knowing the density of alcohol and the density of water, mark their values on the graph paper. Measure the length between the above densities, and hence calibrate the rest of scale.
MAJOR DRAWBACKS :
1. It is not very sensitive and hence cannot record minor changes in density.
2. If placed in too dense liquid, it does not float in an upright position, because of increased upthrust.

