CBSE Class 10 Answered
what is acid base and salt
Asked by luckylucky39382 | 02 Oct, 2020, 22:37: PM
Definition of Acid:
Acid is a proton donor, and electron acceptor.
For example: HCl → H+ + Cl-
Definition of Base:
Base is proton acceptor and electron donor.
For example: NH3 + H+ → NH4+
Definition of Salt
Salt is an ionic compound which dissociates in water to yield a positive ion other than the hydrogen ion (H+) and a negative ion other than the hydroxyl ion (OH−).
For example, NaCl
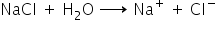
Answered by Ramandeep | 05 Oct, 2020, 13:49: PM
CBSE 10 - Science
Asked by sony.lucky455 | 02 Apr, 2024, 15:23: PM
CBSE 10 - Science
Asked by sai418581 | 17 Dec, 2023, 11:38: AM
CBSE 10 - Science
Asked by mssatish2427 | 10 Dec, 2023, 20:14: PM
CBSE 10 - Science
Asked by shivalaxmi0205 | 04 Sep, 2023, 16:46: PM
CBSE 10 - Science
Asked by Sunita | 28 Jul, 2022, 16:48: PM
CBSE 10 - Science
Asked by tejasgavit38.10dgatl | 21 Oct, 2021, 21:27: PM
CBSE 10 - Science
Asked by begankshetrimayum | 04 Aug, 2021, 22:52: PM
CBSE 10 - Science
Asked by sabitasinha180 | 16 May, 2021, 13:13: PM
CBSE 10 - Science
Asked by pritamkumar91999 | 10 Mar, 2021, 14:35: PM


