ICSE Class 9 Answered
A vessel with a negligible heat capacity contains 1000 g of ice at 0 degree celcius. In to it is poured 100 g of water at 100 degree celcius. What would be the result at the end of the experiment ? ( Sp. heat capacity of water = 4.2 J/g/c and sp. latent heat of ice = 336 J/g )
Asked by nisha_vini29 | 14 Jul, 2016, 11:52: PM
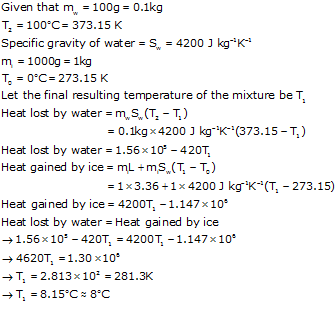
Answered by Yashvanti Jain | 15 Jul, 2016, 02:03: PM

