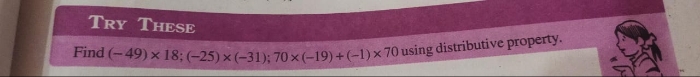Doubts and Solutions
OR
CBSE X - English
Asked by ayankhan893897 | 14 May, 2024, 07:33: PM
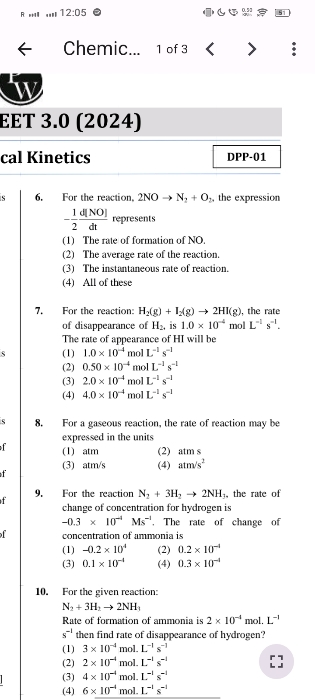
NEET NEET - Chemistry
Asked by rajputmayank04297 | 14 May, 2024, 07:04: PM
CBSE VII - Maths
Asked by sudhabalubeauty | 14 May, 2024, 06:30: PM
CBSE VI - Social Studies
Asked by babulkarmakar7 | 14 May, 2024, 05:39: PM
ICSE VIII - Maths
Asked by Karanraj8210 | 14 May, 2024, 02:27: PM
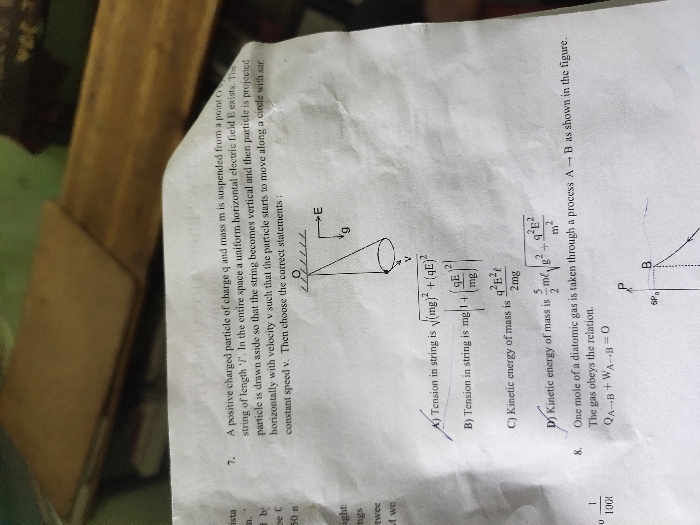
JEE Main - Maths
Asked by pallapothujoshna | 14 May, 2024, 01:59: PM