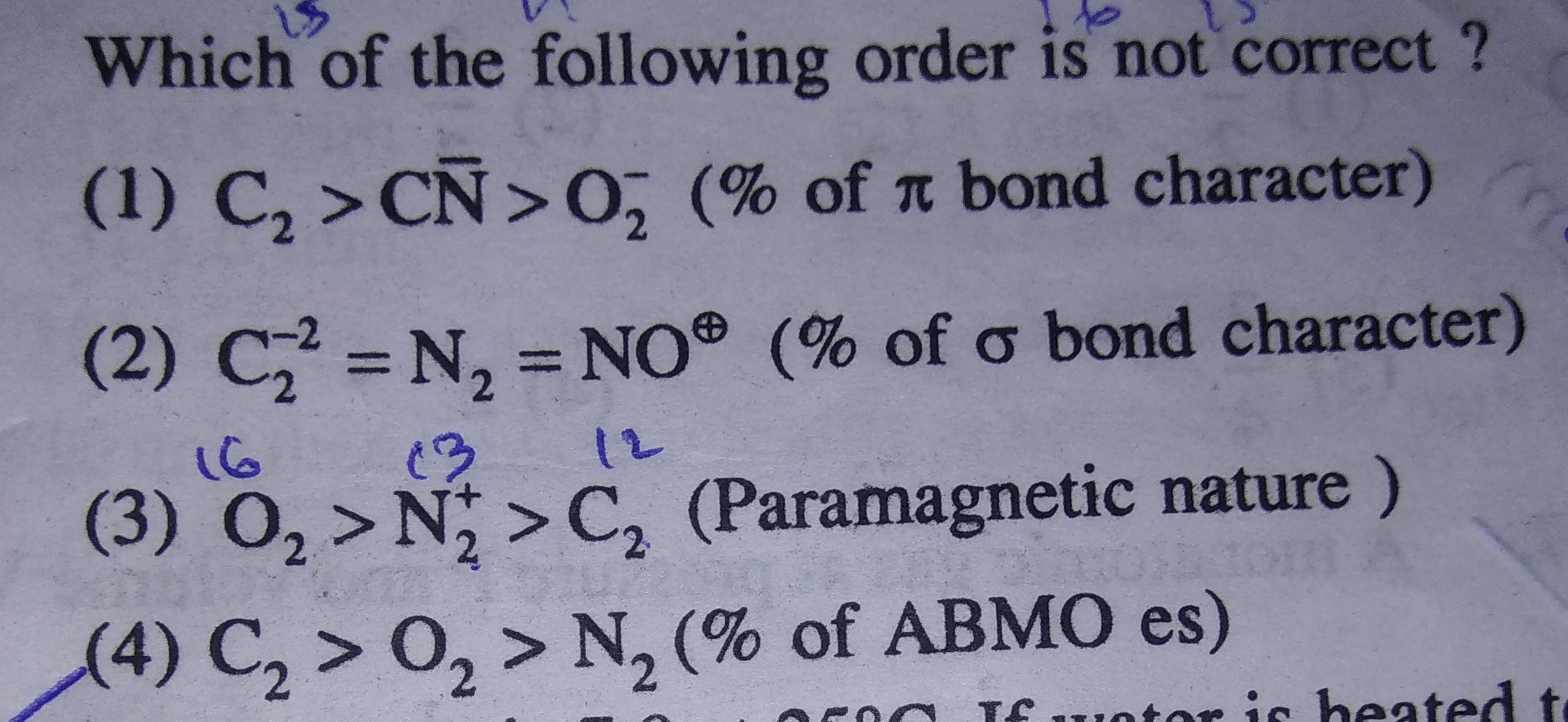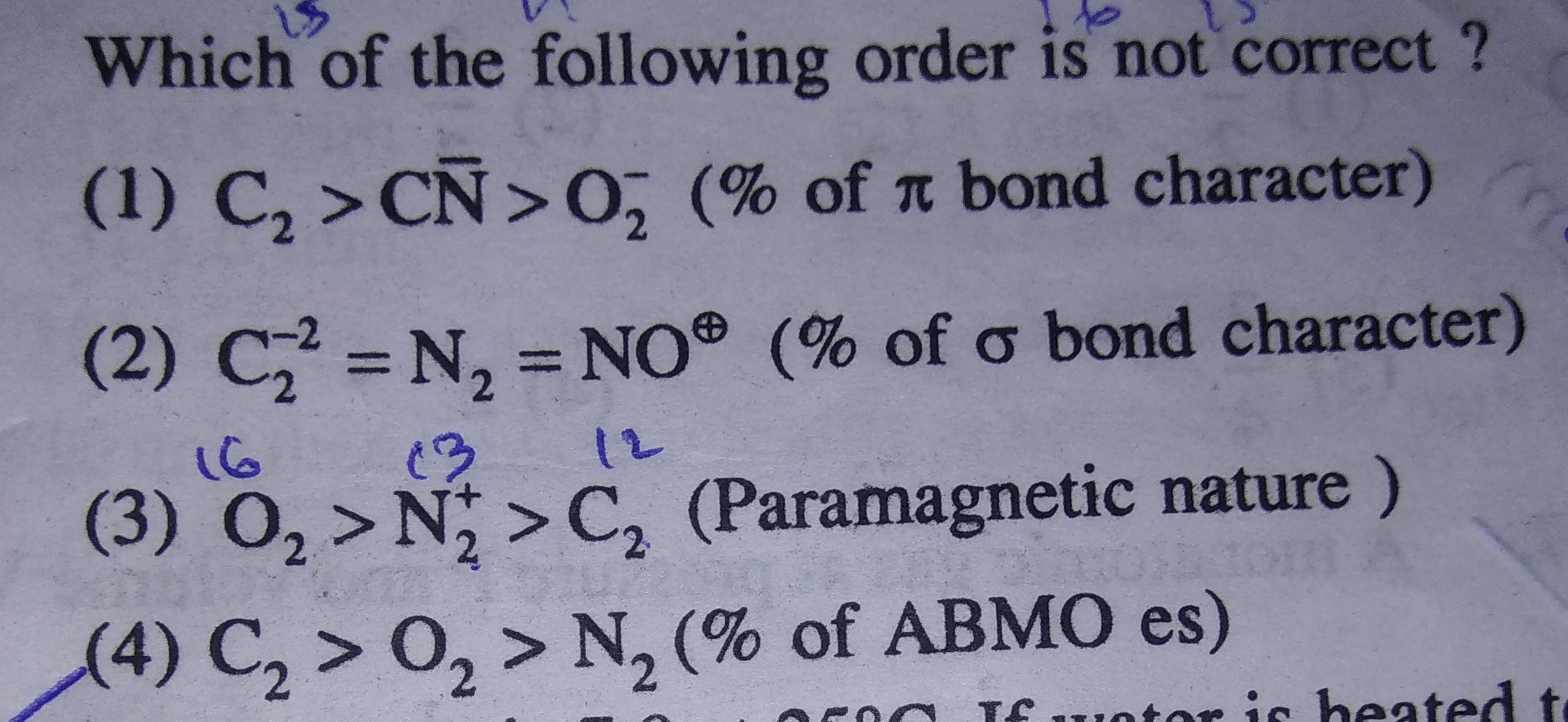(1) In C2 - 2 pi bonds are present. No sigma bond is present . So % pi character=100%
In CN- -2 pi bonds are present and one sigma bond is present, so % pi character=66.6%
In O2- - 1 PI AND 1 sigma bond is present, so % pi character=50%
So option (1) is correct.
(2) in all three molecules 1 sigma and 3 pi bonds are presrent so % of pi bond character is same.
(3) Number of electrons in
The electronic configuration of C2 is (σ2s)2 (σ*2s)2 (2px)2 (2py)2
Electronic configuration of N2+: σ1s2, σ*1s2, σ2s2, σ*2s2, {π2py2, π2pz2}, σ2px1
Electronic configuration of O2: σ1s2, σ*1s2, σ2s2, σ*2s2, σ2px2, {π2py2, π2pz2}, {π*2py1, π*2pz1}
So O2 have 2 unpaird electrons , C2 have none and N+2 have 1.
So given order of paramagnetic is right beaacuse if more no. of unpaired electrons increases than paramagnetic property also increases.
(4)
The electronic configuration of C2 is (σ2s)2 (σ*2s)2 (2px)2 (2py)2
Electronic configuration of N2: σ1s2, σ*1s2, σ2s2, σ*2s2, {π2py2, π2pz2}, σ2px2
Electronic configuration of O2: σ1s2, σ*1s2, σ2s2, σ*2s2, σ2px2, {π2py2, π2pz2}, {π*2py1, π*2pz1}
% antibonding Molecular orbital is
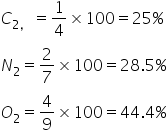
So this option is incorrect