The correct option is (d)....Provide the solution and the reason and justification with it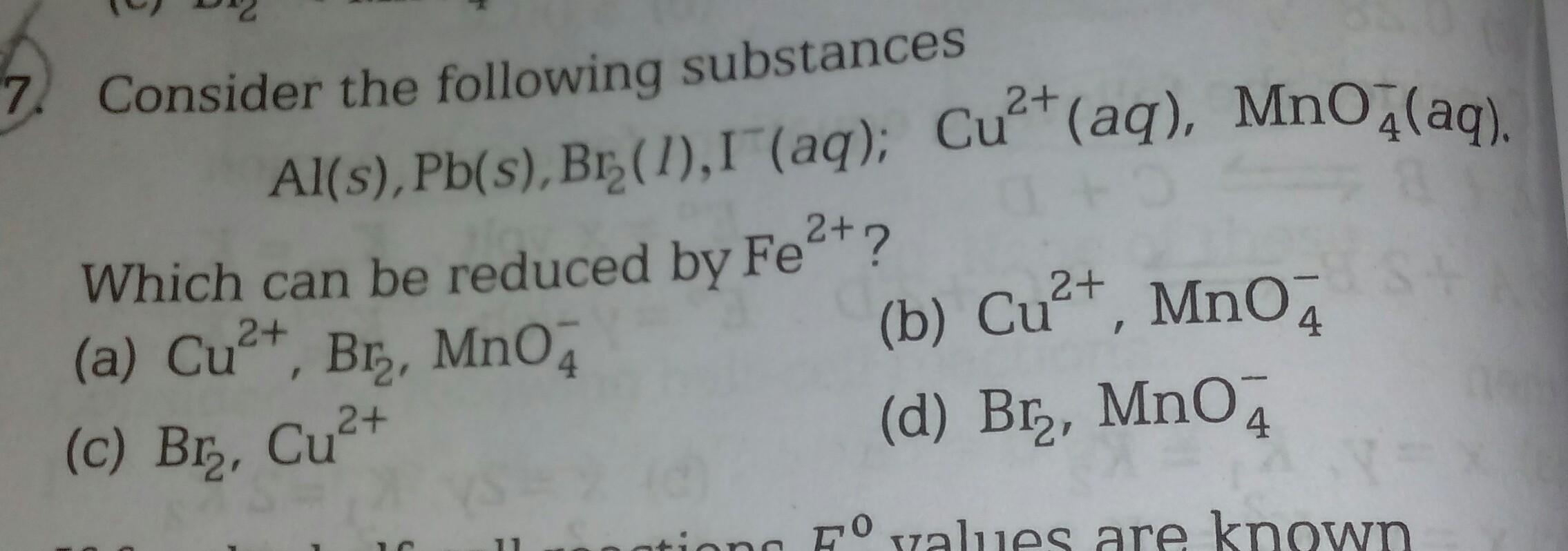
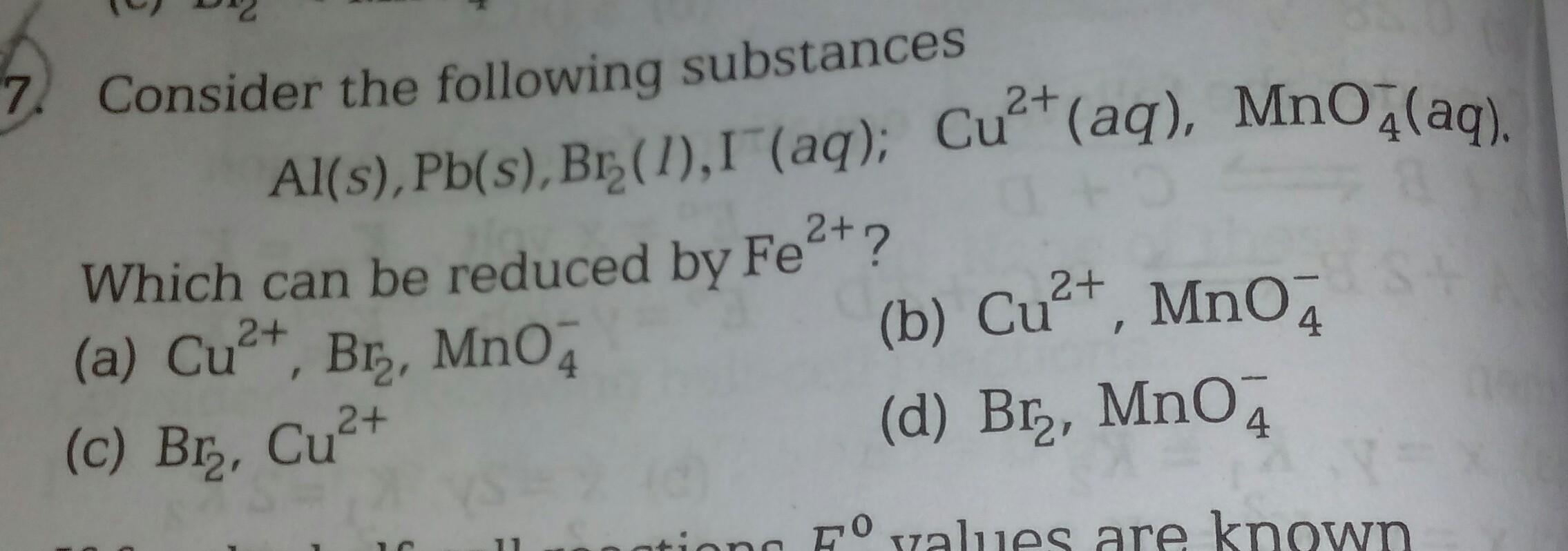
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
You have rated this answer /10
