Q)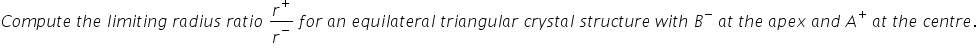
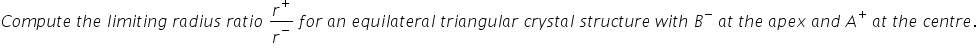
For each coordination number (CN), there is a limiting radius ratio that describes the condition that describes the condition of closest packing.
Regards
Topperlearning Team.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
You have rated this answer /10
