PLease solve the below question with a detailed solution and a proper justification for the method of solving adopted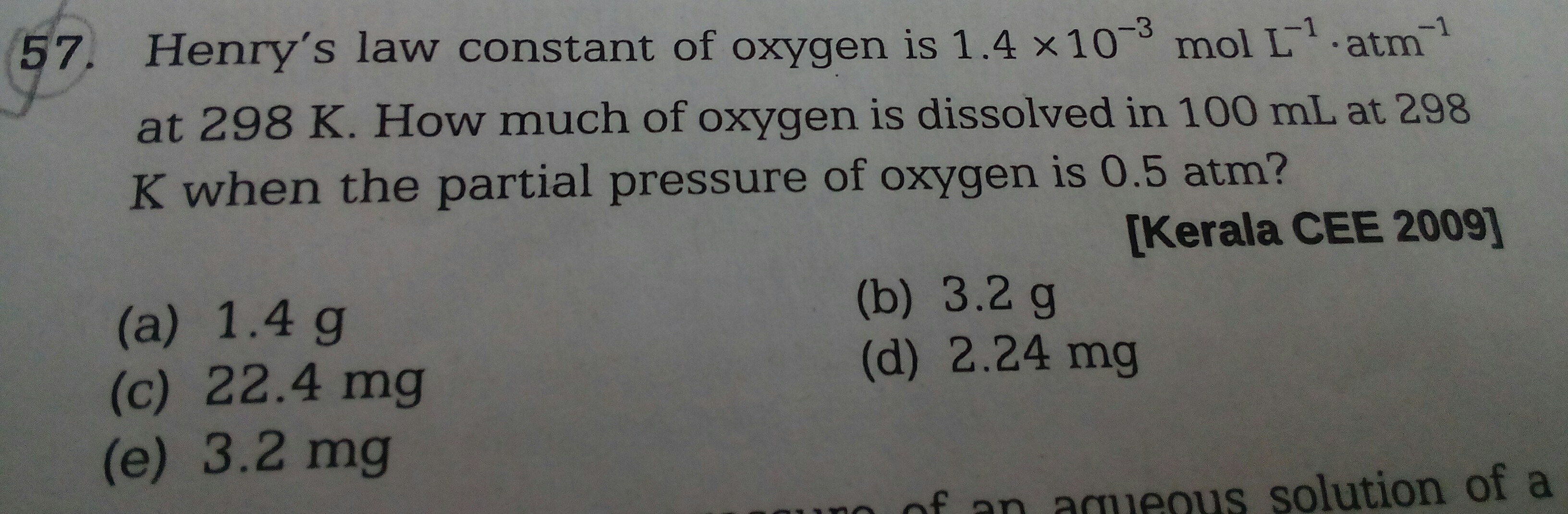
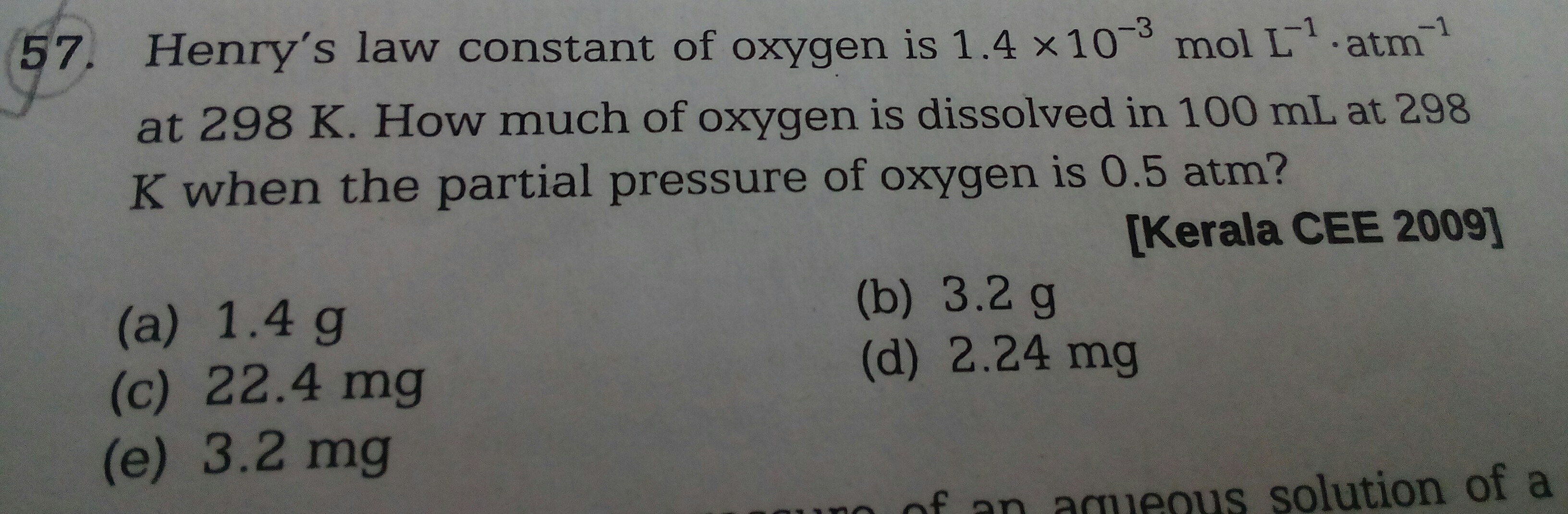
Data:
KH = 1.4 x 10-3 mol L-1
P =0.5 atm
Solution:
KH = C/P
1.4 x 10-3 = C/0.5
C =1.4 x 10-3x 0.5
No. of moles in oxygen in 100 ml= 1.4 x 10-3x 0.5 x 100/1000=1.4 x10-4 x 0.5
Wt. of oxygen in 100 ml = 1.4 x10-4 x 0.5 x32 = 2.24x 10-3g= 2.24mg
Hence the correct option is (d) 2.24 mg
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
You have rated this answer /10
