Please explain
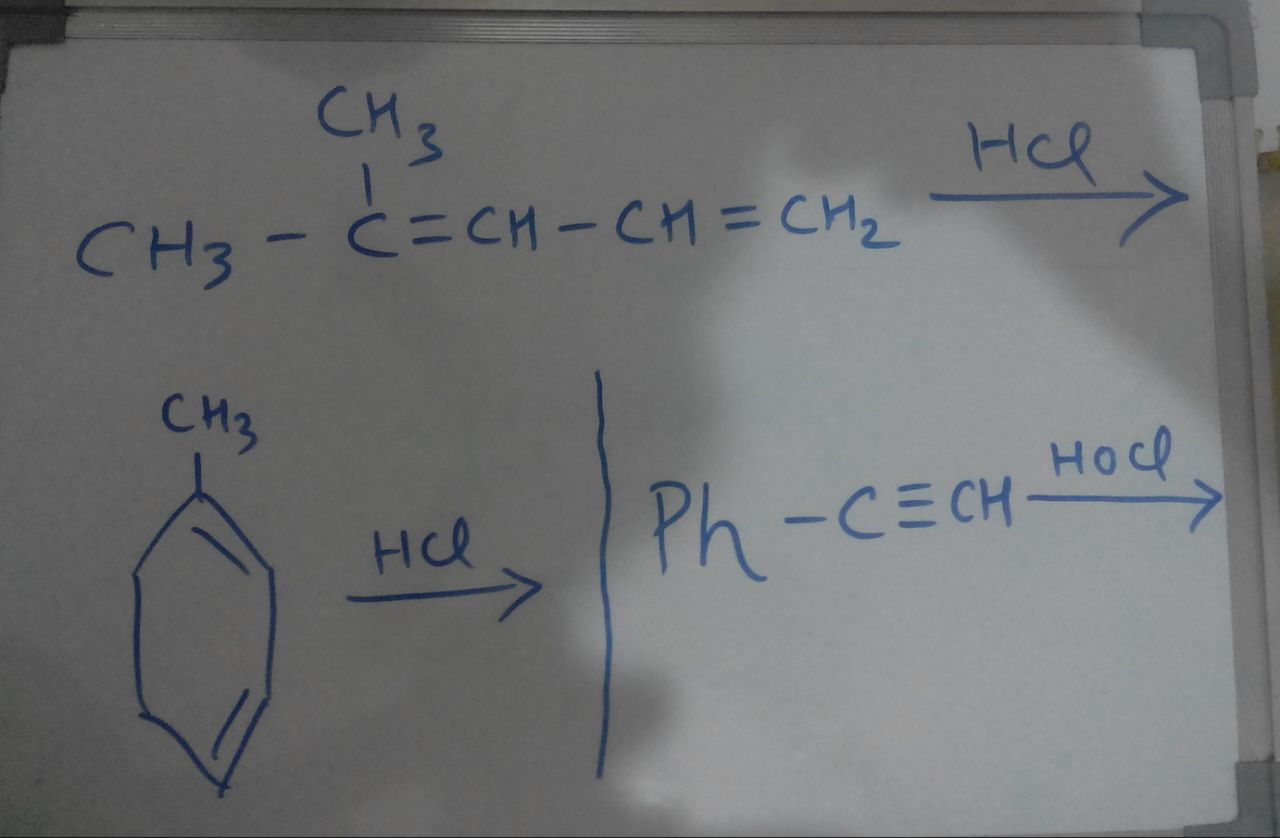


- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
You have rated this answer /10
Browse free questions and answers by Chapters
- 1 Classification of Elements and Periodicity in Properties
- 2 Chemical Bonding and Molecular Structure
- 3 States of Matter
- 4 Thermodynamics
- 5 Equilibrium
- 6 Hydrogen
- 7 Hydrocarbons
- 8 Environmental Chemistry
- 9 Solutions
- 10 Chemical Kinetics
- 11 Surface Chemistry
- 12 Biomolecules
- 13 Polymers
- 14 Chemistry in Everyday Life
- 15 Gravitation
- 16 Electromagnetic Waves
- 17 Communication Systems
- 18 Laws of Motion
- 19 Current Electricity
- 20 Cell : The Unit of Life
- 21 Respiration in Plants
- 22 Work, Energy and Power
- 23 Animal Kingdom
- 24 Atomic Structure
- 25 Kinematics
- 26 Evolution
- 27 Ecosystem
- 28 Chemical Thermodynamics
- 29 Redox Reactions and Electrochemistry
- 30 p-Block Elements
- 31 d - and f - Block Elements
- 32 Some Basic Principles of Organic Chemistry
- 33 Organic Compounds Containing Halogens
- 34 Organic Compounds Containing Oxygen
- 35 Organic Compounds Containing Nitrogen
- 36 Physics and Measurement
- 37 Rotational Motion
- 38 Properties of Solids and Liquids
- 39 Kinetic Theory of Gases
- 40 Oscillations and Waves
- 41 Electrostatics
- 42 Magnetic Effects of Current and Magnetism
- 43 Electromagnetic Induction and Alternating Currents
- 44 Optics
- 45 Dual Nature of Matter and Radiation
- 46 Atoms and Nuclei
- 47 Electronic Devices
- 48 The Living World
- 49 Co-ordination Compounds
- 50 Biological Classification
- 51 Reproduction in Organisms
- 52 Sexual Reproduction in Flowering Plants
- 53 Human Reproduction
- 54 Reproductive Health
- 55 Principles of Inheritance and Variation
- 56 Molecular Basis of Inheritance
- 57 Biotechnology : Principles and Processes
- 58 Biotechnology and its Applications
- 59 Human Health and Disease
- 60 Microbes in Human Welfare
- 61 Strategies for Enhancement in Food Production
- 62 Organisms and Populations
- 63 Biodiversity and Conservation
- 64 Environmental Issues
- 65 Plant Kingdom
- 66 Morphology of Flowering Plants
- 67 Anatomy of Flowering Plants
- 68 Structural Organisation in Animals
- 69 Cell Cycle and Cell Division
- 70 Mineral Nutrition
- 71 Photosynthesis in Higher Plants
- 72 Plant Growth and Development
- 73 Digestion and Absorption
- 74 Breathing and Exchange of Gases
- 75 Body Fluids and Circulation
- 76 Excretory Products and their Elimination
- 77 Locomotion and Movement
- 78 Neural Control and Coordination
- 79 Chemical Coordination and Integration
- 80 Transport in Plants
- 81 Purification and Characterisation of Organic Compounds
- 82 s-Block Element (Alkali and Alkaline Earth Metals)
- 83 Solid State
- 84 Some Basic Concepts in Chemistry
- 85 General Principles and Processes of Isolation of Metals
- 86 Principles Related to Practical Chemistry
