I don't want to know the solution but I just want to know how to proceed with this numerical from atomic structure
Question: it has been found that gaseous iodine molecules dissociate into iodine stomach after absorption of light at wavelength 4995 A. The energy required to dissociate 1 mole of iodine molecules is?
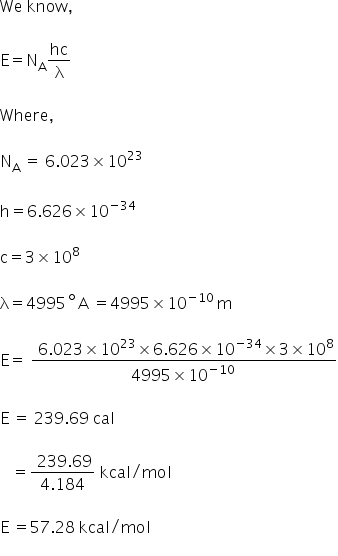
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
You have rated this answer /10
