|
For reaction A(g) + B(g) |
| (1) | 20% | (2) | 40% | (3) | 60% | (4) | 4% |
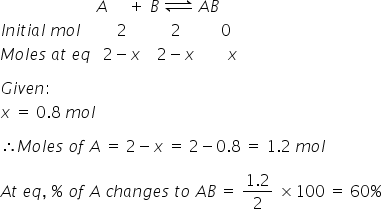
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
You have rated this answer /10
- Biology Question Answers for ICSE Class 12 Science
- Chemistry Question Answers for ICSE Class 12 Science
- Economics Question Answers for ICSE Class 12 Science
- Hindi Question Answers for ICSE Class 12 Science
- Maths Question Answers for ICSE Class 12 Science
- Physics Question Answers for ICSE Class 12 Science
Browse free questions and answers by Chapters
- 1 The Solid State
- 2 Solutions
- 3 Electrochemistry
- 4 Chemical Kinetics
- 5 Surface Chemistry
- 6 Isolation of Elements
- 7 The p-Block Elements
- 8 The d-Block and f-Block Elements
- 9 Coordination Compounds
- 10 Haloalkanes and Haloarenes
- 11 Alcohols, Phenols and Ethers
- 12 Aldehydes, Ketones and Carboxylic Acids
- 13 Amines
- 14 Biomolecules
- 15 Polymers
- 16 Chemistry in Everyday Life
 AB(g) we start with 2 moles of A and B each. At equilibrium 0.8 moles of AB is formed. Then how much of A changes to AB :-
AB(g) we start with 2 moles of A and B each. At equilibrium 0.8 moles of AB is formed. Then how much of A changes to AB :-