Class 10 SELINA Solutions Chemistry Chapter 5 - Mole Concept And Stoichiometry
Mole Concept And Stoichiometry Exercise Ex. 5A
Solution 1
(a)2CO + O2![]() 2CO2
2CO2
2 V 1 V 2 V
2 V of CO requires = 1V of O2
so, 100 litres of CO requires = 50 litres of O2
(b) 2H2 + O2 ![]() 2H2O
2H2O
2 V 1V 2V
From the equation, 2V of hydrogen reacts with 1V of oxygen
so 200cm3 of Hydrogen reacts with = 200/2= 100 cm3
Hence, the unreacted oxygen is 150 - 100 = 50cm3 of oxygen.
Solution 2
This experiment supports Gay lussac's law of combining volumes.
Since the unchanged or remaining O2 is 58 cc so, used oxygen 106 - 58 = 48cc
According to Gay lussac's law, the volumes of gases reacting should be in a simple ratio.
CH4+2O2![]() CO2 + 2H2O
CO2 + 2H2O
1 V2 V
24 cc48 cc
i.e. methane and oxygen react in a 1:2 ratio.
Solution 3
2C2H2+ 5O2![]() 4CO2 + 2H2O (l)
4CO2 + 2H2O (l)
2 V5 V4 V
From equation, 2 V of C2H2 requires = 5 V of O2
So, for 400ml C2H2 , O2 required = 400 ![]() 5/2 =1000 ml
5/2 =1000 ml
Similarly, 2 V of C2H2 gives = 4 V of CO2
So, 400ml of C2H2 gives CO2 = 400 ![]() 4/2 = 800ml
4/2 = 800ml
Solution 4
Balanced chemical equation:
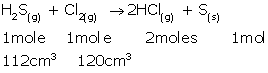
(i)At STP, 1 mole gas occupies 22.4 L.
As 1 mole H2S gas produces 2 moles HCl gas,
22.4 L H2S gas produces 22.4 × 2 = 44.8 L HCl gas.
Hence, 112 cm3 H2S gas will produce 112 × 2 = 224 cm3 HCl gas.
(ii) 1 mole H2S gas consumes 1 mole Cl2 gas.
This means 22.4 L H2S gas consumes 22.4 L Cl2 gas at STP.
Hence, 112 cm3 H2S gas consumes 112 cm3 Cl2 gas.
120 cm3 - 112 cm3 = 8 cm3 Cl2 gas remains unreacted.
Thus, the composition of the resulting mixture is 224 cm3HCl gas + 8 cm3 Cl2 gas.
Solution 5
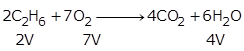
From the equation, 2V of ethane reacts with 7V oxygen.
So, 300 cc of ethane reacts with ![]()
Hence, unused O2 = 1250 - 1050 = 200 cc
From 2V of ethane, 4V of CO2 is produced.
So, 300 cc of ethane will produce ![]()
Solution 6
C2H4+3O2![]() 2CO2 + 2H2O
2CO2 + 2H2O
1V 3V
11litre 33 litre
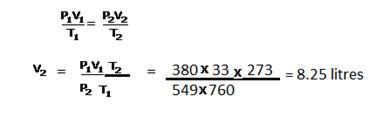
Solution 7
CH4 + 2Cl2 ![]() CH2Cl2 +2HCl
CH2Cl2 +2HCl
1 V2 V1 V2 V
From equation, 1V of CH4 gives = 2 V HCl
so, 40 ml of methane gives = 80 ml HCl
For 1V of methane= 2V of Cl2 required
So, for 40ml of methane = 40 ![]() 2 = 80 ml of Cl2
2 = 80 ml of Cl2
Solution 8
C3H8 + 5O2 ![]() 3CO2 + 4H2O
3CO2 + 4H2O
1 V5 V3 V
From equation, 5 V of O2 required = 1V of propane
so, 100 cm3 of O2will require = 20 cm3 ofpropane
Solution 9
2NO + O2![]() 2NO2
2NO2
2 V1 V2 V
From equation, 1V of O2 reacts with = 2 V of NO
200cm3 oxygen will react with = 200 ![]() 2 =400 cm3 NO
2 =400 cm3 NO
Hence, remaining NO is 450 - 400 = 50 cm3
NO2 produced = 400cm3 because 1V oxygen gives 2 V NO2
Total mixture = 400 + 50 = 450 cm3
Solution 10
i. 6 litres of hydrogen and 4 litres of chlorine when mixed, results in the formation of 8 litres of HCl gas.
ii. When water is added to it, it results in the formation of hydrochloric acid. Chlorine acts as a limiting agent leving behind only 2 litres of hydrogen gas.
iii. Therefore, the volume of the residual gas will be 2 litres.
Solution 11
4NH3 + 5O2![]() 4NO + 6H2O
4NO + 6H2O
4 V5 V4 V
9 litres of reactants gives 4 litres of NO
So, 27 litres of reactants will give = 27 ![]() 4/9 = 12 litres of NO
4/9 = 12 litres of NO
Solution 12
H2 + Cl2 ![]() 2HCl
2HCl
1V1V2 V
Since 1 V hydrogen requires 1 V of oxygen and 4cm3 of H2 remained behind so the mixture had composition: 16 cm3 hydrogen and 16 cm3 chlorine.
Therefore Resulting mixture is H2 =4cm3,HCl=32cm3
Solution 13
CH4 + 2O2 ![]() CO2 + 2H2O
CO2 + 2H2O
1 V2 V1 V
2C2H2 + 5O2 ![]() 4CO2 + 2H2O
4CO2 + 2H2O
2 V5 V4 V
From the equations, we can see that
1V CH4requires oxygen =2 V O2
So, 10cm3 CH4 will require =20 cm3 O2
Similarly 2 V C2H2 requires = 5 V O2
So, 10 cm3 C2H2 will require= 25 cm3 O2
Now, 20 V O2 will be present in 100 V air and 25 V O2 will be present in 125 V air ,so the volume of air required is 225cm3
Solution 14
C3H8 + 5O2![]() 3CO2 + 4H2O
3CO2 + 4H2O
2C4H10 + 13O2 ![]() 8CO2 + 10H2O
8CO2 + 10H2O
60 ml of propane (C3H8) gives 3 ![]() 60 = 180 ml CO2
60 = 180 ml CO2
40 ml of butane (C4H10) gives = 8 ![]() 40/2 = 160 ml of CO2
40/2 = 160 ml of CO2
Total carbon dioxide produced = 340 ml
So, when 10 litres of the mixture is burnt = 34 litres of CO2 is produced.
Solution 15
2C2H2(g) + 5O2(g)![]() 4CO2(g)+ 2H2O(g)
4CO2(g)+ 2H2O(g)
4 V CO2 is collected with 2 V C2H2
So, 200cm3 CO2 will be collected with = 100cm3 C2H2
Similarly, 4V of CO2 is produced by 5 V of O2
So, 200cm3 CO2 will be produced by = 250 ml of O2
Solution 16
According to Avogadro's law, equal volumes of gases contain equal no. of molecules under similar conditions of temperature and pressure. This means more volume will contain more molecules and least volume will contain least molecules.
So,
(a) 5 litres of hydrogen has greatest no. of molecules with the maximum volume.
(b) 1 litre of SO2 contains the least number of molecules since it has the smallest volume.
Solution 17
|
Gas |
Volume (in litres) |
Number of molecules |
|
Chlorine |
10 |
x/2 |
|
Nitrogen |
20 |
x |
|
Ammonia |
20 |
X |
|
Sulphur dioxide |
5 |
x/4 |
Solution 18
(i) According to Avogadro's law, under the same conditions of temperature and pressure, equal volumes of different gases have the same number of molecules.
As 150 cc of gas A contains X molecules, 150 cc of gas B also contains X molecules.
So, 75 cc of B will contain X/2 molecules.
(ii) The problem is based on Avogadro's law.
Mole Concept And Stoichiometry Exercise Ex. 5B
Solution 1
(a) (2N)28 + (8H)8 + (Pt)195 + (6Cl)35.5 x 6 = 444
(b) KClO3 = (K)39 + (Cl)35.5 + (3O)48 = 122.5
(c) (Cu)63.5 + (S)32 + (4O)64 + (5H2O)5 x 18 = 249.5
(d) (2N)28 + (8H)8 + (S)32 + (4O)64 = 132
(e) (C)12 + (3H)3 + (C)12 + (2O)32 + (Na)23 = 82
(f) (C)12 + (H)1+ (3Cl)3 x 35.5 = 119.5
(g) (2N)28 + (8H)8 + (2Cr)2 x 51.9+ (7O)7 x 16 = 252
Solution 2
(a) No. of molecules in 73 g HCl = 6.023 x1023 x 73/36.5(mol.
mass of HCl)
= 12.04 x 1023
(b) Weight of 0.5 mole of O2 is = 32(mol. Mass of O2) x 0.5=16 g
(c) No. of molecules in 1.8 g H2O = 6.023 x 1023 x 1.8/18
= 6.023 x 1022
(d) No. of moles in 10g of CaCO3 = 10/100(mol. Mass CaCO3)
= 0.1 mole
(e) Weight of 0.2 mole H2 gas = 2(Mol. Mass) x 0.2 = 0.4 g
(f) No. of molecules in 3.2 g of SO2 = 6.023 x 1023x 3.2/64
= 3.023 x 1022
Solution 3
Molecular mass of H2O is 18, CO2 is 44, NH3 is 17 and CO is 28
So, the weight of 1 mole of CO2 is more than the other three.
Solution 4
4g of NH3 having minimum molecular mass contain maximum molecules.
Solution 5
a) No. of particles in s1 mole = 6.023 x 1023
So, particles in 0.1 mole = 6.023 x 10 23 x 0.1 = 6.023 x 1022
b)1 mole of H2SO4 contains =2 x 6.023 x 1023
So, 0.1 mole of H2SO4 contains =2 x 6.023 x 1023 x0.1
= 1.2x1023 atoms of hydrogen
c)111g CaCl2 contains = 6.023 x 1023 molecules
So, 1000 g contains = 5.42 x 1024molecules
Solution 6
(a) 1 mole of aluminium has mass = 27 g
So, 0.2 mole of aluminium has mass = 0.2 x 27 = 5.4 g
(b) 0.1 mole of HCl has mass = 0.1 x 36.5(mass of 1 mole)
= 3.65 g
(c) 0.2 mole of H2O has mass = 0.2 x 18 = 3.6 g
(d) 0.1 mole of CO2 has mass = 0.1 x 44 = 4.4 g
Solution 7
(a) 5.6 litres of gas at STP has mass = 12 g
So, 22.4 litre (molar volume) has mass =12 x 22.4/5.6
= 48g(molar mass)
(b)1 mole of SO2 has volume = 22.4 litres
So, 2 moles will have = 22.4 x 2 = 44.8 litre
Solution 8
(a) 1 moleof CO2 contains O2 = 32g
So, CO2 having 8 gm of O2 has no. of moles = 8/32 = 0.25 moles
(b) 16 g of methane has no. of moles = 1
So, 0.80 g of methane has no. of moles = 0.8/16 = 0.05 moles
Solution 9
(a) 6.023 x 10 23 atoms of oxygen has mass = 16 g
So, 1 atom has mass = 16/6.023 x 1023 = 2.656 x 10-23 g
(b) 1 atom of Hydrogen has mass = 1/6.023 x 1023 = 1.666 x 10-24
(c) 1 molecule of NH3 has mass = 17/6.023 x1023 = 2.82 x 10-23 g
(d) 1 atom of silver has mass = 12 × 1022 /6.023 x 1023 = 12/60 = 1/5 = 0.2 g
(e) 1 molecule of O2 has mass = 32/6.023 x 1023 = 5.314 x 10-23 g
(f) 0.25 gram atom of calcium has mass = 0.25 x 40 = 10g
Solution 10
(a) 0.1 mole of CaCO3 has mass =100(molar mass) x 0.1=10 g
(b) 0.1 mole of Na2SO4.10H2O has mass = 322 x 0.1 = 32.2 g
(c) 0.1 mole of CaCl2 has mass = 111 x 0.1 = 11.1g
(d) 0.1 mole of Mg has mass = 24 x 0.1 = 2.4 g
Solution 11
(a) 1molecule of Na2CO3.10H2O contains oxygen atoms = 13
So, 6.023 x1023 molecules (1mole) has atoms=13 x 6.023 x 1023
So, 0.1 mole will have atoms = 0.1 x 13 x 6.023 x 1023 =7.8x1023
(b) Given Na = 4.6 gm
At. mass = 23
No. of gram atoms of Na= Mass of Na
= 4.6
23
= 0.2 gms
(c) 32 g of oxygen gas = 1 mole
1 gram of oxygen gas = 1/32 mole
Given that 12 g of oxygen gas
No: of moles = given mass / molar mass
= 12/32 = 0.375 mole
Solution 12
3.2 g of S has number of atoms = 6.023 x1023 x 3.2 /32
= 0.6023 x 1023
So, 0.6023 x 1023 atoms of Ca has mass=40 x0.6023x1023/6.023
x1023
= 4g
Solution 13
(a) No. of atoms = 52 x 6.023 x1023 = 3.131 x 1025
(b) 4 amu = 1 atom of He
so, 52 amu = 13 atoms of He
(c) 4 g of He has atoms =6.023 x1023
So, 52 g will have = 6.023 x 1023 x 52/4 = 7.828 x1024 atoms
Solution 14
Molecular mass of Na2CO3 = 106 g
106 g has 2 x 6.023 x1023 atoms of Na
So, 5.3g will have = 2 x 6.023 x1023x 5.3/106=6.022 x1022 atoms
Number of atoms of C = 6.023 x1023 x 5.3/106 = 3.01 x 1022 atoms
And atoms of O = 3 x 6.023 x 1023 x 5.3/106= 9.03 x1022 atoms
Solution 15
(a) 60 g urea has mass of nitrogen(N2) = 28 g
So, 5000 g urea will have mass = 28 x 5000/60 = 2.33 kg
(b) 64 g has volume = 22.4 litre
So, 320 g will have volume = 22.4 x 320/64=112 litres
Solution 16
(a) Vapour density of carbon dioxide is 22, it means that 1 molecule of carbon dioxide is 22 heavier than 1 molecule of hydrogen.
(b) Vapour density of Chlorine atom is 35.5.
Solution 17
22400 cm3 of CO has mass = 28 g
So, 56 cm3 will have mass = 56 x 28/22400 = 0.07 g
Solution 18
18 g of water has number of molecules = 6.023 x 1023
So, 0.09 g of water will have no. of molecules = 6.023 x 1023 x 0.09/18 = 3.01 x 1021molecules
Solution 19
(a) No. of moles in 256 g S8 = 1 mole
So, no. of moles in 5.12 g = 5.12/256 = 0.02 moles
(b) No. of molecules = 0.02 x 6.023 x 1023 = 1.2 x 1022 molecules
No. of atoms in 1 molecule of S= 8
So, no. of atoms in 1.2 x 1022 molecules = 1.2 x 1022 x 8
= 9.635x 1022 molecules
Solution 20
Atomic mass of phosphorus P = 30.97 g
Hence, molar mass of P4 = 123.88 g
If phosphorus is considered as P4 molecules,
then 1 mole P4 ≡ 123.88 g
Therefore, 100 g of P4 = 0.807 g
Solution 21
(a) 308 cm3 of chlorine weighs = 0.979 g
So, 22400 cm3 will weigh = gram molecular mass
= 0.979 x 22400/308 =71.2 g
(b) 2 g(molar mass) H2 at 1 atm has volume = 22.4 litres
So, 4 g H2 at 1 atm will have volume = 44.8 litres
Now, at 1 atm(P1) 4 g H2 has volume (V1) = 44.8 litres
So, at 4 atm(P2) the volume(V2) will be = ![]()
(c) Mass of oxygen in 22.4 litres = 32 g(molar mass)
So, mass of oxygen in 2.2 litres = 2.2 x 32/22.4=3.14 g
Solution 22
No. of atoms in 12 g C = 6.023 x1023
So, no. of carbon atoms in 10-12 g = 10-12 x 6.023 x1023/12
= 5.019 x 1010 atoms
Solution 23
Given:
P= 1140 mm Hg
Density = D = 2.4 g / L
T = 273 0C = 273+273 = 546 K
M = ?
We know that, at STP, the volume of one mole of any gas is 22.4 L
Hence we have to find out the volume of the unknown gas at STP.
First, apply Charle’s law.
We have to find out the volume of one litre of unknown gas at standard temperature 273 K.
V1= 1 L T1 = 546 K
V2=? T2 = 273 K
V1/T1 = V2/ T2
V2 = (V1 x T2)/T1
= (1 L x 273 K)/546 K
= 0.5 L
We have found out the volume at standard temperature. Now we have to find out the volume at standard pressure.
Apply Boyle’s law.
P 1 = 1140 mm Hg V1 = 0.5 L
P2 = 760 mm Hg V2 = ?
P1 x V1 = P2 x V2
V2 = (P1 x V1)/P2
= (1140 mm Hg x 0.5 L)/760 mm Hg
= 0.75 L
Now, 22.4 L is the volume of 1 mole of any gas at STP, then 0.75 L is the volume of X moles at STP
X moles = 0.75 L / 22.4 L
= 0.0335 moles
The original mass is 2.4 g
n = m / M
0.0335 moles = 2.4 g / M
M = 2.4 g / 0.0335 moles
M= 71.6 g / mole
Hence, the gram molecular mass of the unknown gas is 71.6 g
Solution 24
1000 g of sugar costs = Rs. 40
So, 342g(molar mass) of sugar will cost=342x40/1000=Rs. 13.68
Mole Concept And Stoichiometry Exercise Ex. 5C
Solution 1
(a) CH
(b) C2H6O
(c) CH
(d) CH2O
Solution 2
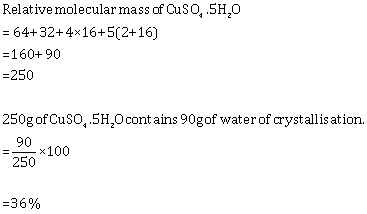
Solution 3
(a) Molecular mass of Ca(H2PO4)2 = 234
So, % of P = 2 ![]() 31
31 ![]() 100/234 = 26.5%
100/234 = 26.5%
(b) Molecular mass of Ca3(PO4)2 = 310
% of P = 2 ![]() 31
31 ![]() 100/310 = 20%
100/310 = 20%
Solution 4
Molecular mass of KClO3 = 122.5 g
% of K = 39 /122.5 = 31.8%
% of Cl = 35.5/122.5 = 28.98%
% of O = 3 ![]() 16/122.5 = 39.18%
16/122.5 = 39.18%
Solution 5
Element%At. massAtomic ratioSimple ratio
Pb62.5207![]() 1
1
N8.514![]() 2
2
O29.016![]() 6
6
So, Pb(NO3)2is the empirical formula.
Solution 6
In Fe2O3 , Fe = 56 and O = 16
Molecular mass of Fe2O3 = 2 ![]() 56 + 3
56 + 3 ![]() 16 = 160 g
16 = 160 g
Iron present in 80% of Fe2O3 = ![]()
So, mass of iron in 100 g of ore = 56 g
![]() mass of Fe in 10000 g of ore = 56
mass of Fe in 10000 g of ore = 56 ![]() 10000/100
10000/100
= 5.6 kg
Solution 7
For acetylene , molecular mass = 2 ![]() V.D = 2
V.D = 2 ![]() 13 = 26 g
13 = 26 g
The empirical mass = 12(C) + 1(H) = 13 g
n = ![]()
Molecular formula of acetylene= 2 ![]() Empirical formula =C2H2
Empirical formula =C2H2
Similarly, for benzene molecular mass= 2 ![]() V.D = 2
V.D = 2 ![]() 39 = 78
39 = 78
n = 78/13=6
So, the molecular formula = C6H6
Solution 8
|
Element |
% |
Atomic mass |
Atomic ratio |
Simple ratio |
|
H |
17.64 |
1 |
17.64/1 =17.64 |
17.64/5.88 = 3 |
|
N |
82.35 |
14 |
82.35/14 = 5.88 |
5.88/5.88 = 1 |
Solution 9
Element%at. massatomic ratiosimple ratio
C54.5412![]() 2
2
H9.091![]() 4
4
O36.3616![]() 1
1
(a) So, its empirical formula= C2H4O
(b) empirical formula mass = 44
Since, vapour density = 44
So, molecular mass = 2 ![]() V.D = 88
V.D = 88
Or n = 2
so, molecular formula = (C2H4O)2 = C4H8O2
Solution 10
Element%at. massatomic ratiosimple ratio
C26.5912![]() 1
1
H2.221![]() 1
1
O71.1916![]() 2
2
(a) its empirical formula = CHO2
(b) empirical formula mass = 45
Vapour density = 45
So, molecular mass = V.D ![]() 2 = 90
2 = 90
so, molecular formula = C2H2O4
Solution 11
Element%at. massatomic ratiosimple ratio
Cl71.6535.5![]() 1
1
H4.071![]() 2
2
C24.2812![]() 1
1
(a) its empirical formula = CH2Cl
(b) empirical formula mass = 49.5
Since, molecular mass = 98.96
so, molecular formula = (CH2Cl)2 = C2H4Cl2
Solution 12
(a) The g atom of carbon = 4.8/12 = 0.4 and g atom of hydrogen = 1/1=1
(b) ElementGiven massAt. massGram atomRatio
C4.8120.412
H1112.55
So, the empirical formula = C2H5
(c) Empirical formula mass = 29
Molecular mass = V.D ![]() 2 = 29
2 = 29 ![]() 2 = 58
2 = 58
So, molecular formula = C4H10
Solution 13
Since, g atom of Si = given mass/mol. Mass
so, given mass = 0.2 ![]() 28 = 5.6 g
28 = 5.6 g
ElementmassAt. massGram atomRatio
Si5.6280.21
Cl21.335.5![]() 3
3
Empirical formula = SiCl3
Solution 14
% of carbon = 82.76%
% of hydrogen = 100 - 82.76 = 17.24%
|
Element |
% Weight |
Atomic Weight |
Relative No. of Moles |
Simplest Ratio |
|
C |
82.76 |
12 |
82.76/12 = 6.89 |
6.89/6.89 = 1 x 2 = 2 |
|
H |
17.24 |
1 |
17.24/1 = 17.24 |
17.24/6.89 = 2.5 x 2 = 5 |
Empirical formula = C2H5
Empirical formula weight = 2 x 12 + 1 x 5 = 24 + 5 = 29
Vapour Density = 29
Relative molecular mass = 29 x 2 = 58
N = 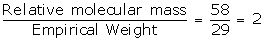
Molecular formula = n x empirical formula
= 2 x C2H5
= C4H10
Solution 15
(a) G atoms of magnesium = 18/24 = 0.75 or g- atom of Mg
(b) G atoms of nitrogen = 7/14= 0.5 or 1/2 g- atoms of N
(c) Ratio of gram-atoms of N and Mg = 1:1.5 or 2:3
So, the formula is Mg3 N2
Solution 16
Barium chloride = BaCl2.x H2O
Ba + 2Cl + x[H2 + O]
=137+ 235.5 + x [2+16]
=[208 + 18x] containswater = 14.8% water in BaCl2.x H2O
=[208 + 18 x] 14.8/100 = 18x
=[104 + 9x] 2148=18000x
=[104+9x] 37=250x
=3848 + 333x =2250x
1917x =3848
x = 2molecules of water
Solution 17
Molar mass of urea; CON2H4 = 60 g
So, % of Nitrogen = 28 ![]() 100/60 = 46.66%
100/60 = 46.66%
Solution 18
Element%At. massAtomic ratioSimple ratio
C42.1123.51
H6.4816.482
O51.42163.21
The empirical formula is CH2O
Since the compound has 12 atoms of carbon, so the formula is
C12 H24 O12.
Solution 19
(a) Now since the empirical formula is equal to vapour density and we know that vapour density is half of the molecular mass i.e. we have n=2 so, the molecular formula is A2B4.
(b) Since molecular mass is 2 times the vapour density, so Mol. Mass = 2 V.D
Empirical formula weight = V.D/3
So, n = molecular mass/ Empirical formula weight = 6
Hence, the molecular formula is A6B6
(c)
Given:
Wt. of the compound: 10.47g
Wt. of metal A: 6.25g
Wt. of non-metal B: 10.47 – 6.25 = 4.22g
|
Element |
mass |
At. Wt. |
Relative no. of atoms |
Simplest ratio |
|
A |
6.25g |
207 |
6.25/207=0.03 |
0.03/0.03=1 |
|
B |
4.22g |
35.5 |
4.26/35.5=0.12 |
0.12/0.03=4 |
Hence, the empirical formula is AB4
Solution 20
Atomic ratio of N = 87.5/14 =6.25
Atomic ratio of H= 12.5/1 = 12.5
This gives us the simplest ratio as 1:2
So, the molecular formula is NH2
Solution 21
Element%at. massatomic ratiosimple ratio
Zn22.65650.3481
H4.8814.8814
S11.15320.3481
O61.32163.8311
Empirical formula of the given compound =ZnSH14O11
Empiricala formula mass = 65.37+32+141+11+16=287.37
Molecular mass = 287
n= Molecular mass/Empirical formula mass = 287/287=1
Molecular formula = ZnSO11H14
=ZnSO4.7H2O
Mole Concept And Stoichiometry Exercise Ex. 5D
Solution 1
(a) 100 g of CaCO3 produces = 164 g of Ca(NO3)2
So, 15 g CaCO3 will produce = 164 ![]() 15/100 = 24.6 g Ca(NO3)2
15/100 = 24.6 g Ca(NO3)2
(b) 1 V of CaCO3 produces 1 V of CO2
100 g of CaCO3 has volume = 22.4 litres
So, 15 g will have volume = 22.4![]() 15/100 = 3.36 litres CO2
15/100 = 3.36 litres CO2
Solution 2
2NH3 + H2SO4 ![]() (NH4)2SO4
(NH4)2SO4
66 g
(a) 2NH3 + H2SO4 ![]() (NH4)2SO4
(NH4)2SO4
34 g98 g132 g
For 132 g (NH4)2SO4 = 34 g of NH3 is required
So, for 66 g (NH4)2SO4 = 66 ![]() 32/132 = 17 g of NH3 is required
32/132 = 17 g of NH3 is required
(b) 17g of NH3 requires volume = 22.4 litres
(c) Mass of acid required, for producing 132g (NH4)2SO4 = 98g
So, Mass of acid required, for 66g (NH4)2SO4 = 66 ![]() 98/132 = 49g
98/132 = 49g
Solution 3
(a) Molecular mass of Pb3O4 = 3 ![]() 207.2 + 4
207.2 + 4 ![]() 16 = 685 g
16 = 685 g
685 g of Pb3O4 gives = 834 g of PbCl2
Hence, 6.85 g of Pb3O4 will give = 6.85 ![]() 834/685 = 8.34 g
834/685 = 8.34 g
(b) 685g of Pb3O4 gives = 71g of Cl2
Hence, 6.85 g of Pb3O4 will give = 6.85 ![]() 71/685 = 0.71 g Cl2
71/685 = 0.71 g Cl2
(c) 1 V Pb3O4produces 1 V Cl2
685g of Pb3O4has volume = 22.4 litres = volume of Cl2 produced
So, 6.85 Pb3O4 will produce = 6.85 ![]() 22.4/685 = 0.224 litres of Cl2
22.4/685 = 0.224 litres of Cl2
Solution 4
Molecular mass of KNO3 = 101 g
63 g of HNO3 is formed by = 101 g of KNO3
So, 126000 g of HNO3 is formed by = 126000 ![]() 101/63 = 202 kg
101/63 = 202 kg
Similarly,126 g of HNO3 is formed by 170 kg of NaNO3
So, smaller mass of NaNO3 is required.
Solution 5
CaCO3 + 2HCl ⟶ CaCl2 + H2O + CO2
100g 73g 2 L
(a)V1 = 2 litres
V2= ?
T1 = (273 + 27) = 300K
T2 = 273K
V1/T1 = V2/T2
V2 = V1T2/T1 = ![]()
Now at STP 22.4 litres of CO2 are produced using CaCO3 = 100g
So, ![]() litres are produced by = (2 × 273 × 100) / (300 × 22.4) = 8.125 g
litres are produced by = (2 × 273 × 100) / (300 × 22.4) = 8.125 g
(b)22.4 litres are CO2 are prepared from acid =73 g
![]() litres are prepared from = (2 × 273 × 73) / (300 × 22.4) =5.9 g
litres are prepared from = (2 × 273 × 73) / (300 × 22.4) =5.9 g
Solution 6
2H2O![]() 2H2 + O2
2H2 + O2
2 V2 V1 V
2 moles of H2O gives = 1 mole of O2
So, 1 mole of H2O will give = 0.5 moles of O2
so, mass of O2 = no. of moles x molecular mass
= 0.5 ![]() 32 = 16 g of O2
32 = 16 g of O2
and 1 mole of O2 occupies volume =22.4 litre
so, 0.5 moles will occupy = 22.4 ![]() 0.5 = 11.2 litres at S.T.P.
0.5 = 11.2 litres at S.T.P.
Solution 7
2Na2O2 + 2H2O![]() 4NaOH + O2
4NaOH + O2
2 V4 V1 V
(a) Mol. Mass of Na2O2 = 2 ![]() 23 + 2
23 + 2 ![]() 16 = 78 g
16 = 78 g
Mass of 2Na2O2= 156 g
156 g Na2O2 gives = 160 g of NaOH (4 ![]() 40 g)
40 g)
So, 1.56 Na2O2 will give = 160 ![]() 1.56/156 = 1.6 g
1.56/156 = 1.6 g
(b) 156 g Na2O2 gives = 22.4 litres of oxygen
So, 1.56 g will give = 22.4 ![]() 1.56/156 = 0.224 litres
1.56/156 = 0.224 litres
= 224 cm3
(c)156 g Na2O2 gives = 32 g O2
So, 1.56 g Na2O2 will give = 32 ![]() 1.56/156
1.56/156
= 32/100 = 0.32 g
Solution 8
2NH4Cl + Ca(OH)2![]() CaCl2+2H2O + 2NH3
CaCl2+2H2O + 2NH3
2 V1 V1 V2 V
Mol. Mass of 2NH4Cl = 2[14 + (1 ![]() 4) + 35.5] = 2[53.5] = 107 g
4) + 35.5] = 2[53.5] = 107 g
(a) 107 g NH4Cl gives = 34 g NH3
So, 21.4 g NH4Cl will give = 21.4 ![]() 34/107 = 6.8 g NH3
34/107 = 6.8 g NH3
(b) The volume of 17 g NH3 is 22.4 litre
So, volume of 6.8 g will be = 6.8 ![]() 22.4/17 = 8.96 litre
22.4/17 = 8.96 litre
Solution 9
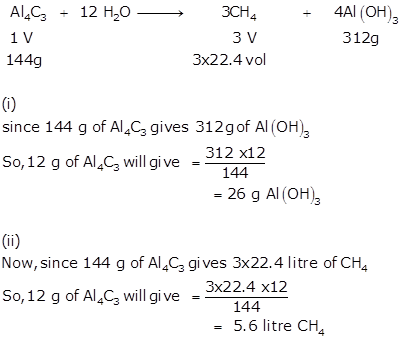
Solution 10
MnO2 + 4HCl ![]() MnCl2 + 2H2O +Cl2
MnCl2 + 2H2O +Cl2
1 V4 V1 V1 V
(a) 1 mole of MnO2 weighs = 87 g (mol. Mass)
So, 0.02 mole will weigh = 87 ![]() 0.02 = 1.74 g MnO2
0.02 = 1.74 g MnO2
(b) 1 mole MnO2 gives = 1 mole of MnCl2
So, 0.02 mole MnO2will give =0.02 mole of MnCl2
(c) 1 mole MnCl2 weighs = 126 g(mol mass)
So, 0.02 mole MnCl2 will weigh = 126 ![]() 0.02 g = 2.52 g
0.02 g = 2.52 g
(d) 0.02 mole MnO2will form =0.02 mole of Cl2
(e) 1 mole of Cl2 weighs = 35.5 g
So, 0.02 mole will weigh = 71 ![]() 0.02 = 1.42 g of Cl2
0.02 = 1.42 g of Cl2
(f) 1 mole of chlorine gas has volume = 22.4 litres
So, 0.02 mole will have volume = 22.4 ![]() 0.02 = 0.448 litre
0.02 = 0.448 litre
(g) 1 mole MnO2requires HCl = 4 mole
So, 0.02 mole MnO2 will require =4 ![]() 0.02 = 0.08 mole
0.02 = 0.08 mole
(h) For 1 mole MnO2 , acid required = 4 mole of HCl
So, for 0.02 mole, acid required = 4 ![]() 0.02 =0.08 mole
0.02 =0.08 mole
Mass of HCl = 0.08 x 36.5 = 2.92 g
Solution 11
N2 + 3H2 ![]() 2NH3
2NH3
28g6g34g
28g of nitrogen requires hydrogen = 6g
2000g of nitrogen requires hydrogen = 6/28 ![]() 2000=3000/7g
2000=3000/7g
So mass of hydrogen left unreacted =1000-3000/7=571.4g of H2
(b)28g of nitrogen forms NH3 = 34g
2000g of N2 forms NH3
= 34/28 ![]() 2000
2000
=2428.6g
Mole Concept And Stoichiometry Exercise Misc. Ex.
Solution A 1
(a) Weight of 1 g atom N = 14 g
So, weight of 2 g atom of N = 28 g
(b) 6.023 x1023 atoms of C weigh = 12 g
So, 3 x1025 atoms will weigh = ![]()
(c) 1 mole of sulphur weighs = 32 g
(d) 7 g of silver
So, 7 grams of silver weighs least.
Solution A 2
Option C is correct.
40 g of NaOH contains 6.023 x 1023 molecules
So, 4 g of NaOH contains = 6.02 x1023 x 4/40
= 6.02 x1022 molecules
Solution A 3
The number of molecules in 18 g of ammonia= 6.02 x1023
So, no. of molecules in 4.25 g of ammonia = 6.02 x1023 x 4.25/18
= 1.5 x 1023
Solution A 4
Mass of gas X =10g
Mass of hydrogen gas= 2
Relative vapour density
=![]() =
=![]() =5
=5
Relative molecular mass of the gas= 2×relative vapour
density = 2×5
=10
Solution A 5
Correct option: (c) molecular mass
Solution:
The relative molecular mass of a gas or vapour is twice its vapour density.
2 × Rel. V. D.= Rel. molecular mass of a gas or vapour
Solution A 6
Correct option: (a) C3H6O3
Solution:
Molecular formula = n × Empirical formula
Where, n = an integer
In the given case, if n = 3, then
C3H6O3 = 3 × CH2O
Solution A 7
Correct option: (b) 29
Solution:
2 × Rel. V. D.= Rel. molecular mass of a gas or vapour
2 × Rel. V. D.= 58
Rel. V. D. = 58 / 2
Rel. V. D. = 29
Solution A 8
Correct option: (d) P3Q6
Solution:
Molecular formula = n × Empirical formula
Where, n = an integer
Also,
Molecular formula mass = n × Empirical formula mass
30 = n × 10
n = 3
Molecular formula = n × Empirical formula
Molecular formula = 3 × PQ2
Molecular formula = P3Q6
Solution A 9
Correct option: (c) 1 : 1
Solution:
Molecular mass of hydrogen is 1 g = 1 mole of hydrogen = 6.023 × 1023 molecules of hydrogen
So, 2 g of hydrogen = 2 moles of hydrogen = 2 × 6.023 × 1023 molecules of hydrogen
Molecular mass of oxygen is 16 g = 1 mole of oxygen = 6.023 × 1023 molecules of oxygen
So, 32 g of oxygen = 2 moles of oxygen = 2 × 6.023 × 1023 molecules of oxygen
Therefore,
Ratio of molecules in 2 g of hydrogen to molecules in 32 g of oxygen is 1:1.
Solution B 1
(a) Moles:1 mole + 2 mole ![]() 1 mole + 2 mole
1 mole + 2 mole
(b) Grams: 42g + 36g![]() 74g+ 4 g
74g+ 4 g
(c) Molecules = 6.02 ![]() 1023 + 12.046
1023 + 12.046 ![]() 1023
1023 ![]() 6.02
6.02 ![]() 1023+ 12.046
1023+ 12.046 ![]() 1023
1023
Solution B 2
(a) One mole of chlorine contains 6.023 x 1023 atoms of chlorine.
(b) Under similar conditions of temperature and pressure, two volumes of hydrogen combined with one volume of oxygen will give two volumes of water vapour.
(c) Relative atomic mass of an element is the number of times one atom of an element is heavier than 1/12 the mass of an atom of carbon-12.
(d) Under similar conditions of temperature and pressure, equal volumes of all gases contain the same number of molecules.
Solution B 3
According to Avogadros law:
Equal volumes of all gases, under similar conditions of temperature and pressure ,contain equal number of molecules.
So, 1 mole of each gas contains = 6.02 ![]() 1023 molecules
1023 molecules
Mol. Mass of H2 (2),O2(32) ,CO2(44),SO2(64),Cl2(71)
(1)Now 2 g of hydrogen contains molecules =6.02 ![]() 1023
1023
So, 8g of hydrogen contains molecules = 8/2 ![]() 6.02
6.02 ![]() 1023
1023
=4 ![]() 6.02
6.02 ![]() 1023 = 4M molecules
1023 = 4M molecules
(2)32g of oxygen contains molecules = 8/32 ![]() 6.02
6.02 ![]() 1023=M/4
1023=M/4
(3)44g of carbon dioxide contains molecules = 8/44 6.02 1023=2M/11
(4)64g of sulphur dioxide contains molecules =6.02 ![]() 1023
1023
So, 8g of sulphur dioxide molecules = 8/64 ![]() 6.02
6.02 ![]() 1023= M/8
1023= M/8
(5)71 g of chlorine contains molecules =6.02 ![]() 1023
1023
So, 8g of chlorine molecules = 8/72 ![]() 6.02
6.02 ![]() 1023 = 8M/71
1023 = 8M/71
Since 8M/71<M/8<2M/11<M/4<4M
Thus Cl2<SO2<CO2<O2<H2
(i)Least number of molecules in Cl2
(ii)Most number of molecules in H2
Solution C 1
(a) The vapour density is the ratio between the masses of equal volumes of gas and hydrogen under the conditions of standard temperature and pressure.
(b) Molar volume is the volume occupied by one mole of the gas at STP. It is equal to 22.4 dm3.
(c) The relative atomic mass of an element is the number of times one atom of the element is heavier than 1/12 times of the mass of an atom of carbon-12.
(d) The relative molecular mass of an compound is the number that represents how many times one moleculae of the substance is heavier than 1/12 of the mass of an atom of carbon-12.
(e) The number of atoms present in 12g (gram atomic mass) of C-12 isotope, i.e. 6.023 x1023 atoms.
(f) The quantity of the element which weighs equal to its gram atomic mass is called one gram atom of that element.
(g) Mole is the amount of a substance containing elementary particles like atoms, molecules or ions in 12 g of carbon-12.
Solution C 2
(a) Gay-Lussac's law states that when gases react, they do so in volumes which bear a simple ratio to one another, and to the volume of the gaseous product, provided that all the volumes are measured at the same temperature and pressure.
(b) Avogadro's law states that equal volumes of all gases under similar conditions of temperature and pressure contain the same number of molecules.
Solution C 3
(a) This is due to Avogadros Law which states Equal volumes of all gases under similar conditions of temperature and pressure contain the same number of molecules.
Now volume of hydrogen gas =volume of helium gas
n molecules of hydrogen =n molecules of helium gas
nH2=nHe
1 mol. of hydrogen has 2 atoms of hydrogen and I molecule of helium has 1 atom of helium
Therefore 2H=He
Therefore atoms in hydrogen is double the atoms of helium.
(b) For a given volume of gas under given temperature and pressure, a change in any one of the variable i.e., pressure or temperature changes the volume.
(c) Inflating a balloon seems violating Boyles law as volume is increasing with increase in pressure. Since the mass of gas is also increasing.
Solution C 4
The empirical formula is the simplest formula, which gives the simplest ratio in whole numbers of atoms of different elements present in one molecule of the compound.
The molecular formula of a compound denotes the actual number of atoms of different elements present in one molecule of a compound.
Solution D 1
Information conveyed by H2O
(1) That H2O contains 2 volumes of hydrogen and 1 volume of oxygen.
(2) That ratio by weight of hydrogen and oxygen is 1:8.
(3) That molecular weight of H2O is 18g.
Solution D 2
(a) stoichiometry measures quantitative relationships and is used to determine the amount of products/reactants that are produced/needed in a given reaction.
(b) The number of atoms in a molecule of an element is called its atomicity. Atomicity of Hydrogen is 2, phosphorus is 4 and sulphur is 8.
(c) N2 means 1 molecule of nitrogen and 2N means two atoms of nitrogen.
N2 can exist independently but 2N cannot exist independently.
Solution D 3
(a) Applications of Avogadro's Law :
(1) It explains Gay-Lussac's law.
(2) It determines atomicity of the gases.
(3) It determines the molecular formula of a gas.
(4) It determines the relation between molecular mass and vapour density.
(5) It gives the relationship between gram molecular mass and gram molecular volume.
(b) According to Avogadro's law under the same conditions of temperature and pressure, equal volumes of different gases have the same number of molecules.
Since substances react in simple ratio by number of molecules, volumes of the gaseous reactants and products will also bear a simple ratio to one another.This what Gay Lussac's Law says.
H2+Cl2?2HCl
1V1V2V(By Gay-Lussacs law)
n moleculesnmolecules2n molecules(By Avogadros law)
Solution D 4
a) This statement means one atom of chlorine is 35.5 times heavier than 1/12 time of the mass of an atom C-12.
b)The value of avogadro's number is 6.023 ![]() 1023
1023
c) The molar volume of a gas at STP is 22.4 dm3 at STP
Solution E 1
From equation: 2H2+ O2![]() 2H2O
2H2O
1 mole of Oxygen gives = 2 moles of steam
so, 0.5 mole oxygen will give = 2 ![]() 0.5 = 1mole of steam
0.5 = 1mole of steam
Solution E 2
3Cu + 8HNO3 ![]() 3Cu (NO3)2+ 4H2O + 2NO
3Cu (NO3)2+ 4H2O + 2NO
1 V8 V3 V2 V
Mol. Mass of 8HNO3 = 8 ![]() 63 = 504 g
63 = 504 g
(a) For 504 g HNO3, Cu required is = 192 g
So, for 63g HNO3Cu required = 192 ![]() 63/504 = 24g
63/504 = 24g
(b) 504 g of HNO3 gives = 2 ![]() 22.4 litre volume of NO
22.4 litre volume of NO
So, 63g of HNO3 gives =2 ![]() 22.4
22.4 ![]() 63/504 =5.6 litre of NO
63/504 =5.6 litre of NO
Solution E 3
(a) 28g of nitrogen = 1mole
So, 7g of nitrogen = 1/28 ![]() 7= 0.25 moless
7= 0.25 moless
(b) Volume of 71 g of Cl2 at STP =22.4 litres
Volume of 7.1 g chlorine =22.4 ![]() 7.1/71=2.24 litre
7.1/71=2.24 litre
(c) 22400cm3 volume have mass =28 g of CO(molar mass)
So, 56cm3 volume will have mass =28 ![]() 56/22400= 0.07 g
56/22400= 0.07 g
Solution E 4
% of N in NaNO3= ![]()
% of N in (NH4)2SO4 = ![]()
% of N in CO(NH2)2 = ![]()
So, highest percentage of N is in urea.
Solution E 5
2H2O![]() 2H2+O2
2H2+O2
2 V2 V1 V
(a) From equation, 2 V of water gives 2 V of H2 and 1 V of O2
where 2 V = 2500 cm3
so, volume of O2 liberated = 2V/V = 1250 cm3
(b)
P1 = 1 Atm.
P2 = 2.5 Atm.
V1 = 2500
V2 = ?
P1 V1 = P2 V2
1 x 2500 = 2.5 x V2
V2 = 2500 x 10/25
V2 = 1000 cm3
(c)
(If volume of H2 = 1000 cm3, temp. is T1, V2 = 2500 and New temp. T2
V1 / T1 = V2 / T2
1000/T1 = 2500/T2
or
T2/T1= 2500/1000
= 2.5
T2 = 2.5 T1
It must be 2.5 times of original temperature.
Solution E 6
Molecular mass of urea=12 + 16+2(14+2) =60g
60g of urea contains nitrogen =28g
So, in 50g of urea, nitrogen present =23.33 g
50 kg of urea contains nitrogen=23.33kg
Solution E 7
% of hydrogen = 20%
% of carbon = 100 - 20 = 80%
|
|
% Weight |
Atomic Weight |
Relative No. of Moles |
Simplest Ratio |
|
C |
80 |
12 |
80/12 = 6.667 |
6.667/6.667 = 1 |
|
H |
20 |
1 |
20/1 = 20 |
20/6.667 = 2.99 ≈ 3 |
Empirical formula = CH3
Empirical formula weight = 1 x 12 + 1 x 3 = 12 + 3 = 15
Vapour Density = 15
Relative molecular mass = 15 x 2 = 30
N = 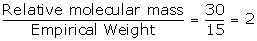
Molecular formula = n x empirical formula
= 2 x CH3
= C2H6
Solution E 8
22400cm3 CO2 has mass = 44g
so, 224 cm3 CO2 will have mass= 0.44 g
Now since CO2 is being formed and X is a hydrocarbon so it contains C and H.
In 0.44g CO2, mass of carbon=0.44-0.32=0.12g=0.01g atom
So, mass of Hydrogen in X = 0.145-0.12 = 0.025g
= 0.025g atom
Now the ratio of C:H is C=1: H=2.5 or C=2 : H=5
i.e. the formula of hydrocarbon is C2H5
(a) C and H
(b) Copper (II) oxide was used for reduction of the hydrocarbon.
(c)
(i) no. of moles of CO2= 0.44/44 = 0.01 moles
(ii) mass of C = 0.12 g
(iii) mass of H = 0.025 g
(iv) The empirical formula of X = C2H5
Solution E 9
Mass of X in the given compound =24g
Mass of oxygen in the given compound =64g
So total mass of the compound =24+64=88g
% of X in the compound = 24/88100 = 27.3%
% of oxygen in the compound=64/88 100 =72.7%
Element%At. MassAtomic ratioSimplest ratio
X27.31227.3/12=2.271
O72.71672.2/16=4.542
So simplest formula = XO2
Solution E 10
(a) V.D = ![]()
(b) Molecular mass = 17(V.D) x 2= 34g
Solution E 11
(a) CO2+C![]() 2CO
2CO
1 V1 V2 V
12 g of C gives =44.8 litre volume of CO
So, 3 g of C will give = 11.2 litre of CO
(b) 2CO + O2![]() 2CO2
2CO2
2 V1 V2 V
(i) 2 V CO requires oxygen = 1 V
so, 24 cm3 CO will require = 24/2 =12 cm3
(ii) 2 x 22400 cm3 CO gives = 2 x 22400 cm3 CO2
so, 24cm3 CO will give = 24 cm3 CO2
Solution E 12
![]()
Molecular weight of ![]() =
=![]()
=328g
Molecular weight of CaO =2(40+16)
=112g
a. 328g of Ca(NO3)2 liberates 4 moles of NO2
328g of Ca(NO3)2 liberates ![]() L of NO2
L of NO2 ![]() 82g will liberate
82g will liberate ![]()
=22.4dm3 of NO2
b. 328 g of calcium nitrate gives 112g of CaO
82 g will give ![]()
=28 g of CaO
Solution E 13
2C8H18 + 25O2![]() 16CO2 + 18H2O
16CO2 + 18H2O
2 V25 V16 V18 V
(i) 2 moles of octane gives = 16 moles of CO2
so, 1 mole octane will give = 8 moles of CO2
(ii) 1 mole CO2 occupies volume = 22.4 litre
so, 8 moles will occupy volume = 8 ![]() 22.4 = 179.2 litre
22.4 = 179.2 litre
(iii) 1 mole CO2 has mass = 44 g
so, 16 moles will have mass = 44 ![]() 16 = 704 g
16 = 704 g
(iv) Empirical formula is C4H9.
Solution E 14
The relative atomic mass of Cl = (35 ![]() 3 + 1
3 + 1 ![]() 37)/4=35.5 amu
37)/4=35.5 amu
Solution E 15
Mass of silicon in the given compound =5.6g
Mass of the chlorine in the given compound=21.3g
Total mass of the compound=5.6g+21.3g=26.9g
% of silicon in the compound = 56/26.9 ![]() 100 = 20.82%
100 = 20.82%
% of chlorine in the compound = 21.2/26.9 ![]() 100 = 79.18%
100 = 79.18%
Element%At. MassAt. RatioSimplest ratio
Si20.822820.82/28=0.741
Cl79.18 35.579.18/35.5=2.233
So the empirical formula of the given compound =SiCl3
Solution E 16
% compositionAtomic ratioSimple ratio
P=38.27%38.27/31 =1.231
H= 2.47%2.47/1 = 2.472
O= 59.26%59.26/16 = 3.703
So, empirical formula is PH2O3 or H2PO3
Empirical formula mass = 31+ 2 ![]() 1 + 3
1 + 3 ![]() 16 = 81
16 = 81
The molecular formula is = H4P2O6, because n = 162/81=2
Solution E 17
a) V1 = 10 litres V2=?
T1= 27+ 273 = 300KT2=273K
P1=700 mmP2 = 760 mm
Using the gas equation
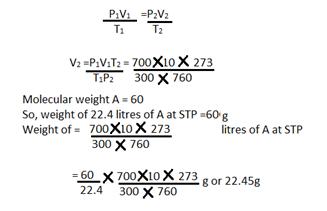
b)

Solution E 18
(a) Molecular mass of CO2 = 12+ 2x16 = 44 g
So, vapour density (V.D) = mol. Mass/2 = 44/2 = 22
V.D = ![]()
![]()
So, mass of CO2 = 22 kg
(b) According to Avogadros law ,equal volumes of all gases under similar conditions of temperature and pressure contain equal number of molecules.
So, number of molecules of carbon dioxide in the cylinder =number of molecules of hydrogen in the cylinder=X
Solution E 19
(a) The volume occupied by 1 mole of chlorine = 22.4 litre
(b) Since PV=constant so, if pressure is doubled; the volume will become half i.e. 11.2 litres.
(c) V1/V2 = T1/T2
22.4/V2 =273/546
V2 = 44.8 litres
(d) Mass of 1 mole Cl2 gas =35.5 x 2 =71 g
Solution E 20
(a) Total molar mass of hydrated CaSO4.xH2O = 136+18x
Since 21% is water of crystallization, so
![]()
So, x = 2 i.e. water of crystallization is 2.
(b) For 18 g water, vol. of hydrogen needed = 22.4 litre
So, for 1.8 g, vol. of H2 needed= 1.8 x 22.4/18 = 2.24 litre
Now 2 vols. of water = 1 vol. of oxygen
1 vol. of water =1/2 vol. of O2 =22.4/2=11.2 lit.
18 g of water = 11.2 lit. of O2
1.8 g of water = 11.2/18 18/10=1.12 lit.
(c) 32g of dry oxygen at STP = 22400cc
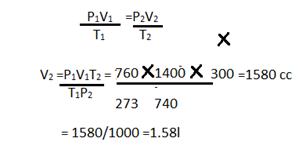
2g will occupy = 224002/32=1400cc
P1=760mmP2 =740mm
V1=1400ccV2 =?
T1 =273 K, T2 = 27 +73 = 300K
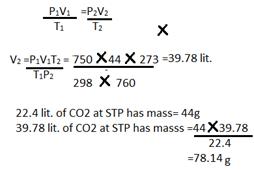
(d) P1= 750mmP2=760mm
V1= 44lit.V2=?
T1= 298KT2=273K
(e) Since 143.5g of AgCl is produced from =58.5 g of NaCl
so, 1.435 g of AgCl is formed by =0.585 g of NaCl
% of NaCl =0.585 x100 = 58.5%
Solution E 21
a.
![]()
For CO2 12+32
i. Molecular mass of sulphuric acid = 2(2+32+64)
= 196
196 g of suphuric acid oxidized 12g of Carbon
49 g of suphuric acid will ![]()
=3 g
ii. 196 g of sulphuric acid gives 2(22.4)
=44.8L
![]() 49 g og sulphuric acid will give
49 g og sulphuric acid will give ![]()
=11.2 L of SO2
b.
i.
|
Element |
% Weight |
Atomic Weight |
Atomic Ratio |
Simplest Ratio |
|
C |
14.4 |
12 |
14.4/12 = 1.2 |
1.2/1.2=1 |
|
H |
1.2 |
1 |
1.2/1 =1.2 |
1.2/1.2=1 |
|
Cl |
84.5 |
35.5 |
84.5/35.5=2.3 |
2.3/1.2=1.9=2 |
Empirical formula = CHCl2
ii.
Empirical formula = CHCl2
Empirical formula weight = 1 x 12 + 1 x 1+(2 x35.5)
= 12 + 1+70
= 83
Relative molecular mass = 168
N = 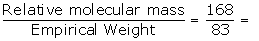 2.02≈2
2.02≈2
Molecular formula = n x empirical formula
= 2 x CHCl2
= C2H2Cl4
Solution E 22
a. Relative molecular mass of [Mg (NO3) 6H2O]
=24+14+(3 x16)+(6 x18)=194
Since, 194g of [Mg (NO3) 6H2O] contains 144g of oxygen
![]() 100g of [Mg (NO3) 6H2O] contains
100g of [Mg (NO3) 6H2O] contains ![]() of oxygen = 74.22%
of oxygen = 74.22%
b. Relative molecular mass of Na2B4O7.10H2O
(23 × 2) + (4 × 11) + (7 × 16) + 10(18) = 382
Since 382g of Na2B4O7.10H2O contains 44g of boron
100g Na2B4O7.10H2O of contains![]() of boron
of boron
=11.5%
c. Relative molecular mass of Ca(H2PO4)2
= 40 + 2(2 + 31 + 64) = 234
Since,234g of Ca(H2PO4)2 contains 62g of phosphorus
100g of Ca(H2PO4)2 contains ![]()
=26.5%
Solution E 23
The given equation is,
2NaOH + CuSO4 → Na2SO4 + Cu(OH)2
Molecular weight of NaOH, sodium hydroxide = 23 + 16 + 1 = 40
Molecular weight of Cu(OH)2 ,
Copper hydroxide = 64 + 16 + 1 + 16 + 1 = 98
Now, 40 g of NaOH is used to precipitate 98 g of Cu(OH)2
Hence, 200 g of NaOH will be used to precipitate (98/40)×200 g of Cu(OH)2 = 490 g of Cu(OH)2
So, 490 g of copper hydroxide would be prepared using 200 g of sodium hydroxide.
Solution E 24
Solid ammonium dichromate decomposes as:
![]()
(a)Molecular mass of ammonium dichromate
= 2(14+4)+104+112
= 252 g
Number of moles=![]()
=![]()
=0.25moles
(b)
252 g of ammonium dichromate gives 22.4 dm3 of N2
63 g of ammonium dichromate gives ![]()
=5.6 L
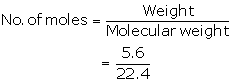
= 0.25 moles
(c)
252 g of ammonium dichromate gives 22.4 dm3 of N2
63 g of ammonium dichromate gives ![]()
= 5.6 L
(d)
Number of moles=![]()
=![]()
=0.25moles
![]()
0.25 moles of ammonium dichromate gives
0.25 moles of N2=7 g
1 mole of H2O =18 g
Therefore, total loss of mass=7+18
=25 g
(e)
252 g of ammonium dichromate gives 152 g of CrO3
63 g of ammonium dichromate gives ![]()
=38 g
Solution E 25
2H2S + 3O2![]() 2H2O + 2SO2
2H2O + 2SO2
2 V3 V2 V
128 g of SO2gives = 2 ![]() 22.4 litres volume
22.4 litres volume
So, 12.8 g of SO2 gives = 2 ![]() 22.4
22.4 ![]() 12.8/128
12.8/128
= 4.48 litre volume
Or one can say 4.48 litres of hydrogen sulphide.
2 ![]() 22.4 litre H2S requires oxygen = 3
22.4 litre H2S requires oxygen = 3 ![]() 22.4 litre
22.4 litre
So, 4.48 litres H2S will require = 6.72 litre of oxygen
Solution E 26
From equation, 2NH3 + 2½ 2 O2![]() 2NO + 3H2O
2NO + 3H2O
When 60 g NO is formed, mass of steam produced = 54 g
So, 1.5 g NO is formed, mass of steam produced = 54 ![]() 1.5/60
1.5/60
=1.35 g
Solution E 27
In 1 hectare of soil, N2 removed = 20 kg
So, in 10 hectare N2 removed = 200 kg
The molecular mass of Ca(NO3)2 =164
Now, 28 g N2 present in fertilizer = 164 g Ca(NO3)2
So, 200000 g of N2 is present in = 164 ![]() 200000/28
200000/28
= 1171.42 kg
Solution E 28
(a) 1 mole of phosphorus atom = 31 g of phosphorus
31 g of P =1 mole of P
6.2g of P = ![]() =0.2 mole of P
=0.2 mole of P
(b) 31 g P reacts with HNO3 = 315 g
so, 6.2 g P will react with HNO3 = 315 ![]() 6.2/31 = 63 g
6.2/31 = 63 g
(c)
Moles of steam formed from 31g phosphorus = 18g/18g = 1mol
Moles of steam formed from 6.2 g phosphorus = 1mol/31g6.2=0.2 mol
Volume of steam produced at STP =0.2 ![]() 22.4 l/MOL=4.48 litre
22.4 l/MOL=4.48 litre
Since the pressure (760mm) remains constant , but the temperature (273+273)=546 is double, the volume of the steam also gets doubled
So,Volume of steam produced at 760mm Hg and 2730C=4.48 ![]() 2=8.96litre
2=8.96litre
Solution E 29
112cm3 of gaseous fluoride has mass = 0.63 g
so, 22400cm3 will have mass = 0.63 ![]() 22400/112
22400/112
= 126 g
The molecular mass = At mass P + At. mass of F
126= 31 + At. Mass of F
So, At. Mass of F = 95 g
But, at. mass of F = 19 so 95/19 = 5
Hence, there are 5 atoms of F so the molecular formula = PF5
Solution E 30
Na2CO3.10H2O ![]() Na2CO3 + 10H2O
Na2CO3 + 10H2O
286 g106 g
So, for 57.2 g Na2CO3.10H2O = 106 ![]() 57.2/286 = 21.2 g Na2CO3
57.2/286 = 21.2 g Na2CO3
Solution E 31
Simple ratio of M = 34.5/56 = 0.616 = 1
Simple ratio of Cl = 65.5/35.5 = 1.845 = 3
Empirical formula = MCl3
Empirical formula mass = 162.5, Molecular mass = 2 ![]() V.D = 325
V.D = 325
So, n = 2
So, molecular formula = M2Cl6
Solution E 32
(i) Element%atomic massatomic ratiosimple ratio
C4.812![]() 1
1
Br95.280![]() 3
3
So, empirical formula is CBr3
(ii) Empirical formula mass = 12 + 3 ![]() 80 = 252 g
80 = 252 g
molecular formula mass = 2 ![]() 252(V.D) = 504 g
252(V.D) = 504 g
n= 504/252 = 2
so, molecular formula = C2Br6
Solution E 33
4N2O + CH4 ![]() CO2 + 2H2O + 4N2
CO2 + 2H2O + 4N2
4 V1 V1 V2 V4 V
2 x 22400 litre steam is produced by N2O = 4 x 22400 cm3
So, 150 cm3 steam will be produced by= 4 ![]() 22400
22400 ![]() 150/2 x 22400
150/2 x 22400
= 300 cm3N2O
Solution E 34
(a) Volume of O2 = V
Since O2 and N2 have same no. of molecules = x
so, the volume of N2 = V
(b) 3x molecules means 3V volume of CO
(c) 32 g oxygen is contained in = 44 g of CO2
So, 8 g oxygen is contained in = 44 x 8/32 = 11 g
(d) Avogadro's law is used in the above questions.
Solution E 35
simple ratio of Na = 42.1/23 = 1.83 = 3
simple ratio of P = 18.9/31 = 0.609 = 1
simple ratio of O = 39/16 = 2.43 = 4
So, the empirical formula is Na3PO4
Solution E 36
CH4 + 2O2 ![]() CO2 + 2H2O
CO2 + 2H2O
1 V2 V1 V2 V
From equation:
22.4 litres of methane requires oxygen = 44.8 litres O2
2H2 + O2 ![]() 2H2O
2H2O
2 V1 V2 V
From equation,
44.8 litres hydrogen requires oxygen = 22.4 litres O2
So, 11.2 litres will require = 22.4 x 11.2/44.8 = 5.6 litres
Total volume = 44.8 + 5.6 = 50.4 litres
Solution E 37
Gram atoms of Pb = 6.21/207=0.03 = 1
Gram atoms of Cl = 4.26/35.5 = 0.12 = 4
So, the empirical formula = PbCl4
Solution E 38
Na2SO4+BaCl2![]() BaSO4+2NaCl
BaSO4+2NaCl
Molecular mass of BaSO4 = 233 g
Now, 233 g of BaSO4 is produced by Na2SO4 = 142 g
So, 6.99 g BaSO4 will be produced by = 6.99 ![]() 142/233 = 4.26
142/233 = 4.26
The percentage of Na2SO4 in original mixture = 4.26 ![]() 100/10
100/10
= 42.6%
Solution E 39
(a) 1 litre of oxygen has mass = 1.32 g
So, 24 litres (molar vol. at room temp.) will have mass = 1.32 x 24
= 31.6 or 32 g
(b) 2KMnO4![]() K2MnO4 + MnO2 + O2
K2MnO4 + MnO2 + O2
316 g of KMnO4 gives oxygen = 24 litres
So, 15.8 g of KMnO4 will give = 24 ![]() 316/15.8 = 1.2 litres
316/15.8 = 1.2 litres
Solution E 40
(a)
(i) The no. of moles of SO2 = 3.2/64 = 0.05 moles
(ii) In 1 mole of SO2, no. of molecules present = 6.02 ![]() 1023
1023
So, in 0.05 moles, no. of molecules = 6.02![]() 1023
1023 ![]() 0.05
0.05
= 3.0 ![]() 1022
1022
(iii) The volume occupied by 64 g of SO2 = 22.4 dm3
3.2 g of SO2 will be occupied by volume= 22.4 ![]() 3.2/64 =1.12 dm3
3.2/64 =1.12 dm3
Solution E 41
(i) D contains the maximum number of molecules because volume is directly proportional to the number of molecules.
(ii) The volume will become double because volume is directly proportional to the no. of molecules at constant temperature and pressure.
V1/V2 = n1/n2
V1/V2 = n1/2n1
So, V2 = 2V1
(iii) Gay lussac's law of combining volume is being observed.
(iv) The volume of D = 5.6 ![]() 4 = 22.4 dm3, so the number of molecules = 6 x 1023 because according to mole concept 22.4 litre volume at STP has = 6 x 10 23 molecules
4 = 22.4 dm3, so the number of molecules = 6 x 1023 because according to mole concept 22.4 litre volume at STP has = 6 x 10 23 molecules
(v) No. of moles of D = 1 because volume is 22.4 litre
so, mass of N2O = 1 ![]() 44 = 44 g
44 = 44 g
Solution E 42
(a) NaCl+NH3+ CO2 + H2O![]() NaHCO3+NH4Cl
NaHCO3+NH4Cl
2NaHCO3 ![]() Na2CO3+H2O + CO2
Na2CO3+H2O + CO2
From equation:
106 g of Na2CO3 is produced by = 168 g of NaHCO3
So, 21.2 g of Na2CO3 will be produced by = 168 ![]() 21.2/106
21.2/106
= 33.6 g of NaHCO3
(b) For 84 g of NaHCO3, requiredvolume of CO2 = 22.4 litre
So, for 33.6 g of NaHCO3, required volume of CO2 = 22.4 x 33.6/84
= 8.96 litre
Solution E 43
(a) NH4NO3![]() N2O+2H2O
N2O+2H2O
1mole1mole2mole
1 V1 V2 V
44.8 litres of water produced by = 22.4 litres of NH4NO3
So, 8.96 litres will be produced by = 22.4 x 8.96/44.8
= 4.48 litres of NH4NO3
So, 4.48 litres of N2O is produced.
(i) 44.8 litre H2O is produced by = 80 g of NH4NO3
So, 8.96 litre H2O will be produced by = 80 x 8.96/44.8
= 16g NH4NO3
(iii) % of O in NH4NO3 = 3x16/80 = 60%
Solution E 44
![]()
Molecular mass of ![]()
![]() =(240) g
=(240) g
Molecular mass of ![]() = 2 × 22.4 = 44.8dm3
= 2 × 22.4 = 44.8dm3
Molecular mass of ![]() = (192)g
= (192)g
240 g of CuO requires 44.8 dm3 of NH3
![]() 120g of CuO will require
120g of CuO will require ![]()
=22.4dm3
Solution E 45
(a) The molecular mass of ethylene(C2H4) is 28 g
No. of moles = 1.4/28 = 0.05 moles
No. of molecules = 6.023 x1023 x 0.05 = 3 x 1022 molecules
Volume = 22.4 x 0.05 = 1.12 litres
(b) Molecular mass = 2 X V.D
S0, V.D = 28/2 = 14
Solution E 46
(a) Molecular mass of Na3AlF6 = 210
So, Percentage of Na = 3x23x100/210 = 32.85%
(b) 2CO + O2![]() 2CO2
2CO2
2 V1 V2 V
1 mole of O2 has volume = 22400 ml
Volume of oxygen used by 2 x 22400 ml CO = 22400 ml
So, Vol. of O2 used by 560 ml CO =22400 x 560/(2 x 22400)
= 280 ml
So, Volume of CO2 formed is 560 ml.
Solution E 47
(a)
i. The combustion reaction ![]()
According to Gay-Lussac's law,
2 volume of acetylene requires 5 volume of oxygen to burn it
![]() 1 volume of acetylene requires 2.5 volume of oxygen to burn it
1 volume of acetylene requires 2.5 volume of oxygen to burn it
![]() 200cm3 requires 2.5×200=500 cm3 of oxygen
200cm3 requires 2.5×200=500 cm3 of oxygen
2 volume of acetylene on combustion gives 4CO2
![]() 1 volume of acetylene on combustion gives 2CO2
1 volume of acetylene on combustion gives 2CO2
![]() 200cc of acetylene on combustion will give 200×2=400cc of CO2
200cc of acetylene on combustion will give 200×2=400cc of CO2
(b) Hydrogen = 12.5%
∴ Nitrogen= 100-12.5= 87.5%
|
Element |
% Weight |
Atomic Weight |
Atomic Ratio |
Simplest Ratio |
|
N |
87.5 |
14 |
87.5/14=6.25 |
6.25/6.25=1 |
|
H |
12.5 |
1 |
12.5/1=12.5 |
12.5/6.25=2 |
The Empirical formula of the compound is NH2
Empirical formula weight =14+2=16
Relative molecular mass =37
N = 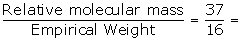 2.3≈2
2.3≈2
Molecular formula = n x empirical formula = 2 x NH2
=N2H4
(c)
Molecules of nitrogen gas in a cylinder = 24 x 1024
Avogadro's number = 6 x 1023
(i) Mass of nitrogen in a cylinder = ![]()
=1120g
(ii) Volume of nitrogen at stp
Volume of 28 g of N2 = 22.4dm3
Volume of 1120g of N2 = ![]() dm3
dm3
=896 dm3
(d) NaCl + AgNO3 ⟶ AgCl + NaNO3
As, 143 g of AgCl is obtained from = 58 g of NaCl
So, 14.3 g of AgCl will be obtained from = 58 × 14.3 / 143 = 5.8 g of NaCl
Weight of commercial NaOH = 30 g
Percentage of NaCl in NaOH = 5.8 × 100 / 30 = 19.33 %
(e) At STP, 100 cm3 of gas weighs = 0.5 g
So, at STP 22400 cm3 of gas will weigh = 0.5 x 22400 / 100 = 112 g
Solution E 48
(a)
(i) C3H8(g) + 5O2(g) ⟶ 3CO2(g) + 4H2O(g)
2C4H10(g) + 13O2(g) + 8CO2(g) + 10H2O(g)
60 ml of propane (C3H8) gives 3 × 60 = 180 ml CO2
40 ml of butane (C4H10) gives = 8 × 40 / 2 = 160 ml of CO2
Total carbon dioxide produced = 340 ml
So, when 10 litres of the mixture is burnt = 34 litres of CO2 is produced.
(ii) Molecular mass of NH4(NO3) =80
H=1, N=14, O=16
% of Nitrogen
As 80 g of NH4(NO3) contains 28 g of nitrogen
![]() 100 g of of NH4(NO3) will contain
100 g of of NH4(NO3) will contain ![]()
= 35%
% of Oxygen
As,80 g of NH4(NO3) contains 48 g of oxygen
![]() 100 g of of NH4(NO3) will contain
100 g of of NH4(NO3) will contain ![]()
= 60%
(b)
(i) Equation for reaction of calcium carbonate with dilute hydrochloric acid:
![]()
(ii) Relative molecular mass of calcium carbonate=100
Mass of 4.5 moles of calcium carbonate
= No. of moles× Relative molecular mass
= 4.5×100
= 450g
(iii) ![]()
As, 100g of calcium carbonate gives 22.4dm3 of CO2
![]() 450 g of calcium carbonate will give
450 g of calcium carbonate will give ![]()
=100.8 L
(iv) Molecular mass of calcium carbonate =100
Relative molecular mass of calcium chloride =111
As 100 g of calcium carbonate gives 111g of calcium chloride
![]() 450 g of calcium carbonate will give
450 g of calcium carbonate will give ![]()
=499.5 g
(v) Molecular mass of HCl=36.5
Molecular mass of calcium carbonate =100
As 100 g of calcium carbonate gives (2×36.5)= 73g of HCl
![]() 450 g of calcium carbonate will give
450 g of calcium carbonate will give ![]()
=328.5g
Number of moles of HCl= ![]()
= ![]()
= 9 moles
Solution E 49
(a)
(i) Atomic mass: S = 32 and O = 16
Molecular mass of SO2=32+(2×16)
=64g
As 64 g of SO2 = 22.4dm3
Then, 320 g of SO2 =![]()
=112 L
(ii) Gay-Lussac's law Gay-Lussac's Law states "When gases react, they do so in volumes which bear a simple ratio to one another and to the volume of the gaseous product, if all the volumes are measured at the same temperature and pressure."
(iii) C3H8 + 5O2→3CO2 + 4H2O
Molar mass of propane = 44
44 g of propane requires 5 × 22.4 litres of oxygen at STP.
8.8 g of propane requires ![]() = 22.4 litres
= 22.4 litres
(b)
(i)
|
Element |
Relative atomic mass |
%Compound |
Atomic ratio |
Simplest ratio |
|
H |
1 |
2.13 |
2.13/1=2.13 |
2 |
|
C |
12 |
12.67 |
12.67/12=1.055 |
1 |
|
Br |
80 |
85.11 |
85.11/80=1 |
1 |
Empirical formula = CH2Br
n(Empirical formula mass of CH2Br) = Molecular mass (2 × VD)
n(12 + 2 + 80) = 94 × 2
n = 2
Molecular formula = Empirical formula × 2
= (CH2Br) × 2
= C2H4Br2
(ii)
1. 1022 atoms of sulphur
6.022 × 1023 atoms of sulphur will have mass = 32 g
1022 atoms of sulphur will have mass = 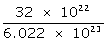
= 0.533 g
2. 0.1 mole of carbon dioxide
1 mole of carbon dioxide will have mass = 44 g
0.1 mole of carbon dioxide will have mass = 4.4 g
Solution E 50
(a) ![]()
(i) Number of moles of phosphorus taken = ![]()
= 0.3 mol
(ii) 1 mole of phosphorus gives 98 gm of phosphoric acid.
So, 0.3 mole of phosphorus gives (0.3 × 98) gm of phosphoric acid
= 29.4 gm of phosphoric acid
(iii) 1 mole of phosphorus gives 112 L of NO![]() gas at STP.
gas at STP.
So, 0.3 mole of phosphorus gives (112 × 0.3) L of
NO![]() gas at STP.
gas at STP.
= 33.6 L of NO![]() gas at STP
gas at STP
(b)
(i) According to the equation
![]()
3 volumes of hydrogen produce 2 volumes of ammonia
67.2 litres of hydrogen produce ![]() = 44.8 L
= 44.8 L
3 volumes of hydrogen combine with 1 volume of ammonia.
67.2 litres of hydrogen combine with ![]() =22.4L Nitrogen left = 44.8 - 22.4 = 22.4 litres
=22.4L Nitrogen left = 44.8 - 22.4 = 22.4 litres
(ii) 5.6 dm3 of gas weighs 12 g
1 dm3 of gas weighs = (12/56) gm
22.4 dm3 of gas weighs = (12/56 × 22.2) gm = 48g
Therefore, the relative molecular mass of gas = 48 gm.
(iii) Molar mass of Mg (NO3)2.6H2O
= 24 × (14 × 2) + (16 × 12) + (1 × 12) = 256 g
Mass percent of magnesium = ![]()
Solution E 51
(a)
(i) ![]()
2 vols. of butane requires O2 = 13 vols
90 dm3 of butane will require O2 = ![]() × 90
× 90
= 585 dm3
(ii) Molecular mass = 2 × Vapour density
So, molecular mass of gas = 2 × 8 = 16 g
As we know, molecular mass or molar mass occupies 22.4 litres.
That is,
16 g of gas occupies volume = 22.4 litres
So, 24 g of gas will occupy volume
= ![]()
(iii) According to Avogadro's law, equal volumes of all gases under similar conditions of temperature and pressure contain the same number of molecules.
So, molecules of nitrogen gas present in the same vessel = X
(b)
(i) ![]()
3 vols. of oxygen require KClO3 = 2 vols.
So, 1 vol. of oxygen will require KClO3 = ![]()
So, 6.72 litres of oxygen will require KClO3
=![]()
22.4 litres of KClO3 has mass = 122.5 g
So, 4.48 litres of KClO3 will have mass
= ![]()
(ii) 22.4 litres of oxygen = 1 mole
So, 6.72 litres of oxygen =![]()
No. of molecules present in 1 mole of O2
= 6.023 × 1023
So, no. of molecules present in 0.3 mole of O2
= 6.023 × 1023 × 0.3
= 1.806 × 1023
(iii) Volume occupied by 1 mole of CO2 at STP = 22.4 litres
So, volume occupied by 0.01 mole of CO2 at STP = 22.4 × 0.01= 0.224 litres
Solution E 52
(a)
(i) ![]()
2 moles of C2H2=4moles of CO2
x dm3 of C2H2 =8.4 dm3 of CO2
x=![]()
=4.2 dm3 of C2H2
(ii) Empirical formula= X2Y
Atomic weight (X)= 10
Atomic weight (Y)= 5
Empirical formula weight = (2 × 10) + 5
=25
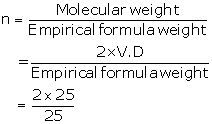
= 2
So, molecular formula = X2Y×2
= X4Y2
(b)
(i) A cylinder contains 68 g of ammonia gas at STP.
Molecular weight of ammonia = 17 g/mole
68 g of ammonia gas at STP =?
1 mole = 22.4 dm3
∴ 4 mole = 22.4 × 4 = 89.6 dm3
(ii) 4 moles of ammonia gas is present in the cylinder.
(iii) 1 mole = 6.023 × 1023 molecules
4 moles = 24.092 × 1023 molecules

