Class 10 FRANK Solutions Chemistry Chapter 10 - Study of Sulphur Compound: Sulphuric Acid
Revise Frank Solutions for ICSE Class 10 Chemistry Chapter 10 Study of Sulphur Compounds: Sulphuric Acid at TopperLearning. Learn how sulphuric acid is prepared with our chapter solutions. Revise terms such as a dehydrating agent, oleum, hygroscopic substance etc. Also, learn to describe reactions that reveal that concentrated sulphuric acid is a dehydrating agent.
With our ICSE Class 10 Chemistry textbook solutions, learn the key differences between concentrated sulphuric acid and dilute sulphuric acid. Practising MCQs from our Frank Solutions is valuable. However, you can also use our Selina Chemistry solutions to prepare well and increase your final exam marks.
Study of Sulphur Compound: Sulphuric Acid Exercise 249
Solution 1
Solution 2

Solution 3

Solution 4

Study of Sulphur Compound: Sulphuric Acid Exercise 250
Solution 5

Solution 6


Solution 7

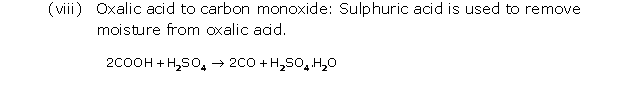
Solution 8

Solution 9

Solution 10

Solution 11


Study of Sulphur Compound: Sulphuric Acid Exercise 251
Solution 12
(i) 2SO2 + O2→ 2SO3
(ii) Dehydration
(iii) SO3
(iv) Fe(OH)3
(v) Vanadium pentoxide
Solution 13
(i) The catalyst used in contact process for the manufacture of H2SO4 is ___________ or _________.
(ii) For the reaction SO2 + O2⇌ SO3 + Heat, the favourable conditions are _________ and __________.
(iii) Oil of vitriol is sulphuric acid.
Solution 1993-1
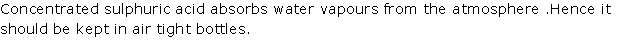
Solution 1994-1

Solution 1994-2
Solution 1994-3

Solution 1995-1
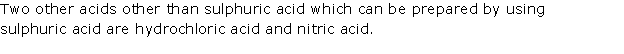
Solution 1995-2

Study of Sulphur Compound: Sulphuric Acid Exercise 252
Solution 1995-3
Solution 1995-4
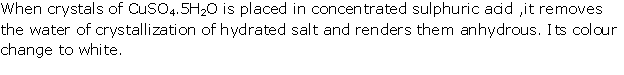
Solution 1995-5
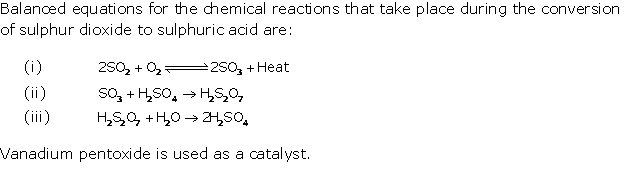
Solution 1996-1

Solution 1998-1
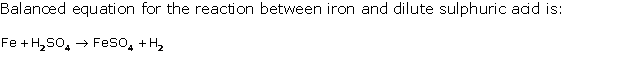
Solution 1998-2

Solution 1998-3

Solution 1999-1
Solution 1999-2

Solution 2000-1
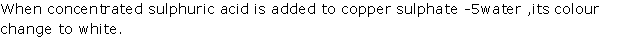
Solution 2002-1
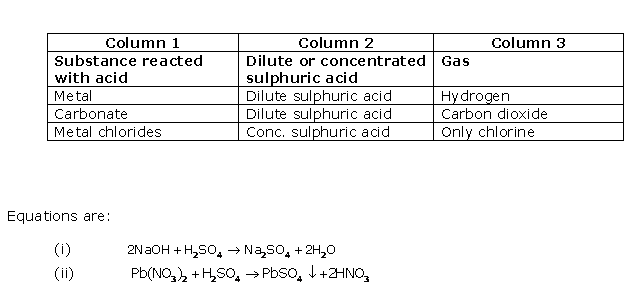
Study of Sulphur Compound: Sulphuric Acid Exercise 253
Solution 2003-1

Solution 2003-2
Solution 2003-3

Solution 2004-1
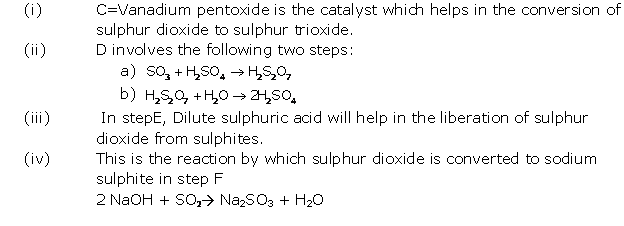
Solution 2005-1

Solution 2005-2

Study of Sulphur Compound: Sulphuric Acid Exercise 254
Solution 2006-1

Solution 2007-1
Solution 2007-2
(a) B
(b) D
(c) C
(d) A
(e) A
Solution 2007-3

Solution 2008-1
Solution 2008-2
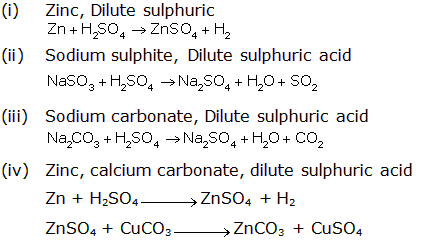
Study of Sulphur Compound: Sulphuric Acid Exercise 255
Solution 2009-1
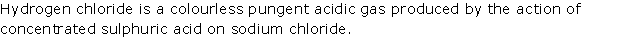
Solution 2010-1
(i) S + H2SO4→ 3SO2 + 2H2O
(ii) ![]()
Solution 2010-2
(i) Litmus turns blue to red, and then gets bleached.
(ii) Dry SO2 has no effect on dry red rose petals.
(iii) Paper turns from pink to white.
Solution 2011-1
Charring of sugar takes place. Sulphuric acid dehydrates sugar leaving behind carbon which is black.
Solution 2011-2
(i) Formation of sulphur dioxide:
S + O2 → SO2
Conversion of SO2 to SO3
![]()
Conversion of sulphur trioxide to oleum:
SO3 + H2SO4→ H2S2O7
Dilution of oleum:
H2S2O7 + H2O → 2H2SO4
(ii)
i. Non-volatile nature
ii. As an oxidising agent
Solution 2013-1
(b) Oxidising agent
Solution 2013-2
(i) When conc. H2SO4 is added to a crystal of hydrated copper sulphate, it removes water of crystallisation from salt.
(ii) C12H22O11 + conc. H2SO4→ 6C + 6H2O
Solution 2014-1
(i) Dehydrating property of sulphuric acid:
H2SO4 has a great affinity for water, and therefore, it acts as a dehydrating agent.
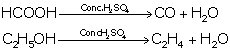
(ii) Acidic nature of sulphuric acid:
It acts as a strong dibasic acid.
![]()
It reacts with metals, metal oxides, metal hydroxides and carbonates to form metallic sulphates and hydrogen at ordinary temperature.
Mg + H2SO4→ MgSO4 + H2↑
CuO + H2SO4 → CuSO4 + H2O
2NaOH + H2SO4→ Na2SO4 + 2H2O
ZnCO3 + H2SO4→ ZnSO4 + H2O + CO2↑
(iii) As a non-volatile acid:
It has a high boiling point, so it is used to prepare volatile acids such as HCl, HNO3 and acetic acid from their salts.
NaCl + H2SO4→ NaHSO4 + HCl
NaNO3 + H2SO4 → NaHSO4 + HNO3
CH3COONa + H2SO4 → NaHSO4 + CH3COOH
Solution 2015-1
(i)
1. Action of sulphuric acid on potassium hydrogen carbonate
2KHCO3 + H2SO4→ K2SO4 + 2H2O + 2CO2↑
2. Action of sulphuric acid on sulphur
S + 2H2SO4→ 3SO2 + 2H2O
(ii) In the contact process for the manufacture of sulphuric acid, the equations for the conversion of sulphur trioxide to sulphuric acid are
SO3 + H2SO4→ H2S2O7
(oleum or pyrosulphuric acid)
H2S2O7 + H2O → 2H2SO4
Study of Sulphur Compound: Sulphuric Acid Exercise 256
Solution 2016-1
(i) B
(ii) A
(iii) C
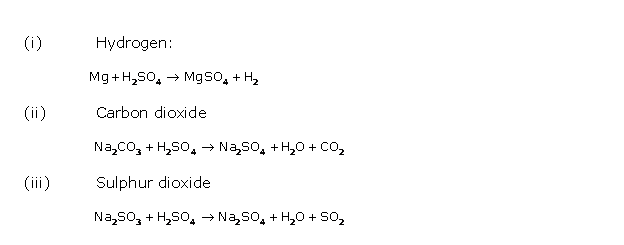
Solution 2017-1
(i) ![]()
(ii) ![]()
(iii) ![]()

