Class 10 FRANK Solutions Chemistry Chapter 3 - Study Of Acids, Bases and Salts
Revise Frank Solutions for ICSE Class 10 Chemistry Chapter 3 Study of Acids, Bases and Salts at TopperLearning. In this Chemistry chapter, study about volatile acids, weak dibasic acids, strong monobasic acids, alkali, base and other topics. Also, learn the various methods to prepare salts such as zinc sulphate, sodium sulphate etc.
In this chapter, learn the right chemical equations to explain reactions like conversion of hydrogen chloride to anhydrous iron (III) chloride. For more Chemistry practice, explore our ICSE Class 10 Chemistry Selina Solutions and other revision resources.
Study Of Acids, Bases and Salts Exercise 70
Solution 1
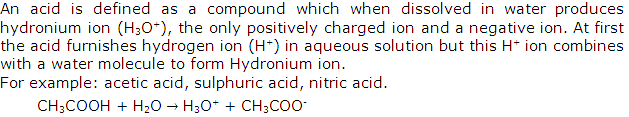
Solution 2
(ii) Nitric acid HNO3
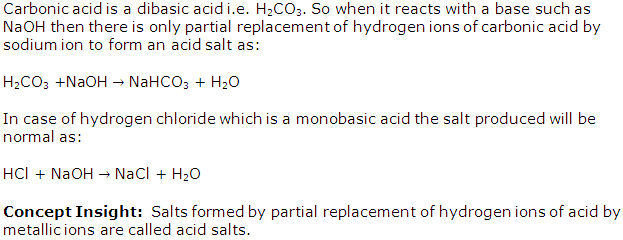
(b) (i) Carbonic acid H2CO3
(ii) Oxalic acid (COOH)2
(c) (i) Sulphuric acid H2SO4
(ii) Hydrogen chloride HCl
(d) (i) Carbonic acid H2CO3
(ii) Acetic acid
Solution 3
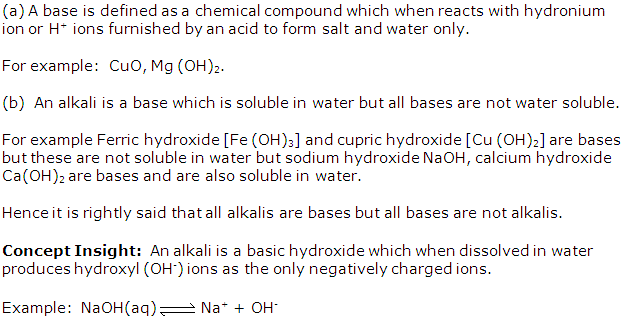
Solution 4
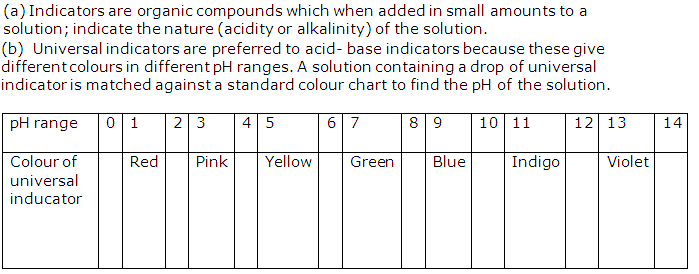
(a) The pH of a solution is defined as the negative logarithm (base 10) of the hydronium ion concentration present in the solution.
pH = -log10 [H3O+]
(b) The three applications of pH scale are:
It is used to determine the acidic or basic nature of the solution.
It is used to determine hydronium ion concentration present in the solution.
It is used to find out neutrality of the solution.
Solution 5
Solution 6
Solution 7
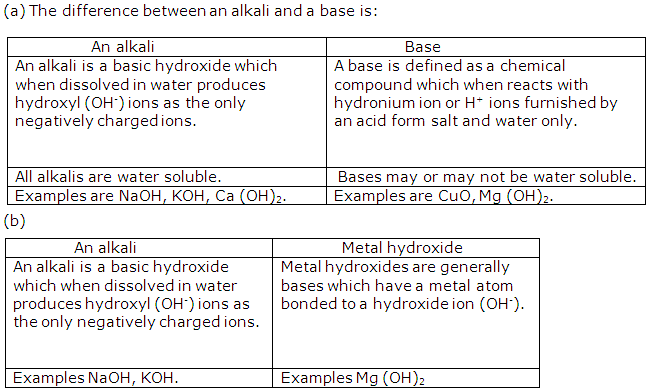
Hydroxide ion/ oxide ion and a metallic ion.
(b) A weak alkali furnishes the ions:
Hydroxide ion and metallic ion and molecules of weak alkali./
(c) An acid in a solution furnishes the ions:
Hydronium / Hydrogen ion and a negative ion.
Solution 8
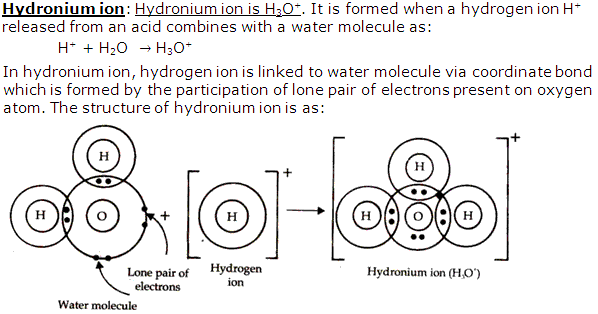
Solution 9
(b) NaOH
(c) CuO
(d) Cu[(OH)2]
(e) H2CO3
(f) Ferric hydroxide [Fe (OH)3].
(g) CuO
(h) NH3
Study Of Acids, Bases and Salts Exercise 71
Solution 10
Solution 11
Solution 12
Strength of an acid measures the ease with which the acid can ionize to produce hydrogen or hydronium ions when dissolved in water. Those acids which can easily ionize to form hydrogen ions are called strong acids while those which can partially ionize to form hydrogen ions are called weak acids.
Strength of an acid depends upon many factors such as:
Molecular structure of the acid
The temperature
Properties of the solvent
Solution 13

Solution 14
Concept Insight: Bases give pink colour with phenolphthalein because a base will abstract two protons from phenolphthalein and the resulting phenolphthalein ion provides pink colour to the solution.
Solution 15
Solution 16
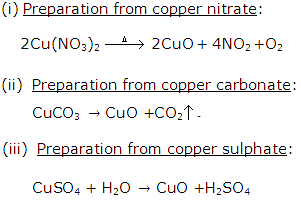
Solution 17
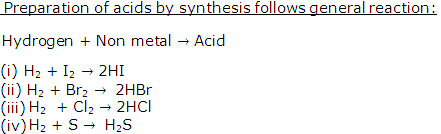
Solution 18
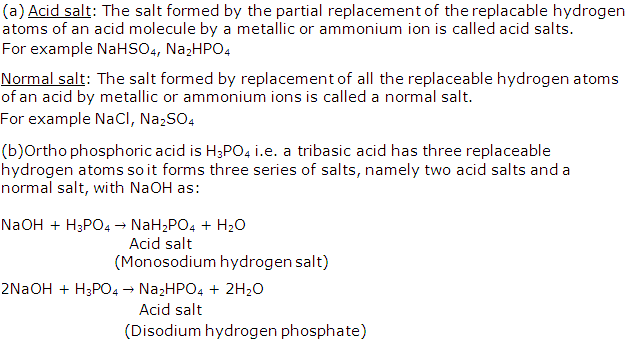
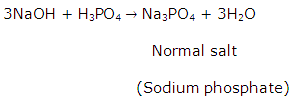
Solution 19
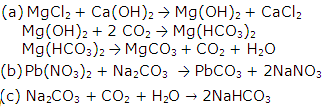
Solution 20
(ii) Hygroscopy: It is the phenomenon by which substances absorb moisture from air, but only sufficiently so as to become wet.
(iii) Water of crystallization: It is the fixed amount of water that is present in a crystal as an integral part of its constitution. Hydrated salts are salts having water of crystallisation.
Solution 21
The substances which exhibit deliquescence are called deliquescent.
For example Caustic soda NaOH, Caustic potash KOH.
Solution 22

Solution 23

Solution 24
(b) (i) Na2CO3.10H2O = Washing soda
(ii) MgSO4.7H2O = Epsom salt
(iii) CuSO4.5H2O = Blue vitriol
(iv) ZnSO4.7H2O = White vitriol
Solution 25
Reactions of SO2:
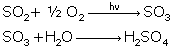
Reactions of oxides of nitrogen:
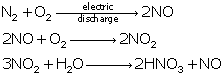
Solution 26
(a) The word acid comes from the latin word acidus meaning 'sour'.
(b) Vinegar is the source of acetic acid.
(c) Magnesia is used in making refractory bricks.
(d) pH scale was introduced by Sorencen in 1909.
(e) Efflorescence is the phenomenon by which hydrated salts on exposure to dry air lose their water of crystallisation and crumble to powder.
Study Of Acids, Bases and Salts Exercise 72
Solution 27
(i) Citric acid
(ii) Food preservative
(iii) Violet
(iv) Calcium chloride
Solution 1996-1
(ii) pH can be decreased by adding an acid such as HCl to it.
If a solution changes colour of litmus from red to blue, it shows that its pH is above 7.
Solution 1996-2
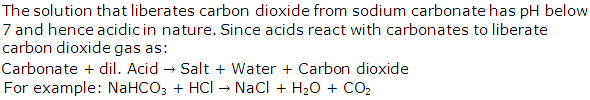
Solution 1996-3
(b) Copper sulphate = Copper oxide and dilute sulphuric acid
(c) Sodium sulphate = Sodium carbonate solution and dilute sulphuric acid
(d) Lead sulphate = Lead carbonate and dilute sulphuric acid
Solution 1997-1
For example: NaHCO3
Solution 1997-2
(ii) pH of pure water is 7 since it does not have any impurities.
(iii) (a) A soluble oxide of A will have pH less than the pH of pure water i.e. below 7.
(b) A solution of 'B' will have more pH than pure water i.e. above 7.
Solution 1997-3
For example: Sodium carbonate Na2CO3 has 10 molecules of water present as water of crystallization Na2CO3.10H2O
(ii) Anhydrous: Hydrous salt on heating lose their water of crystallization, such salts are then called anhydrous.
For example: Na2CO3.10H2O on losing 10 molecules of water forms Na2CO3.
Solution 1997-4

Study Of Acids, Bases and Salts Exercise 73
Solution 1998-1
(i) Water of cystallization.
(ii) White.
(iii) Efflorescence.
(iv) Sodium chloride.
Solution 1998-2
Those acids which ionize partially in aqueous solution and thus they contain ions as well as molecules of the acid. Organic acid such as CH3COOH, is a weak acid.
Solution 1998-3
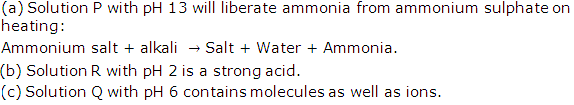
Solution 1998-4
Solution 1999-1

Solution 2000-1
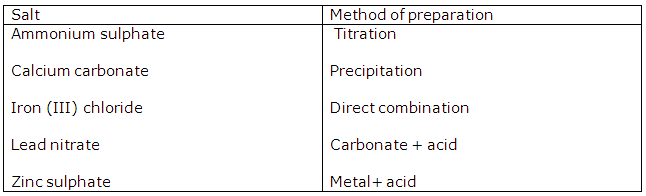
Study Of Acids, Bases and Salts Exercise 74
Solution 2001-1

Solution 2002-1
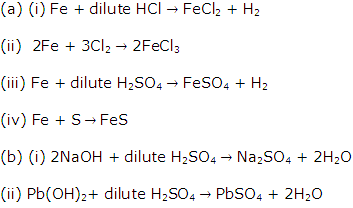
Solution 2003-1
(ii) Acid, metal.
Solution 2003-2
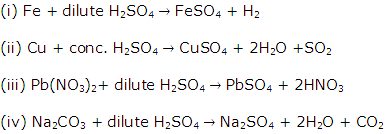
Solution 2004-1
(i) Preparation of copper(II) chloride.
Action of an acid on an oxide or carbonate
(ii) Preparation of iron(III) chloride.
Direct combination
(iii) Preparation of iron (II) chloride.
Action of an acid on a metal
(iv) Preparation of lead (ii) chloride
Precipitation (double decomposition)
(v) Preparation of sodium chloride
Neutralization of an alkali by an acid.
Study Of Acids, Bases and Salts Exercise 75
Solution 2005-1
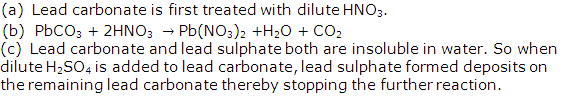
Solution 2005-2
Solution 2005-3
Solution 2006-1
(b) From orange to pink.
(c) From colourless to red.
Solution 2007-1
(ii) Hydroxide
(iii) Salt
(iv) Water
(v) Hydrogen
Solution 2007-2
Study Of Acids, Bases and Salts Exercise 76
Solution 2008-1
(ii) Alkali.
Solution 2009-1
Solution 2009-2
(ii) Solution A.
(iii) Solution B
(iv) Solution of ammonium hydroxide NH4OH is a weak alkali.
Solution 2009-3
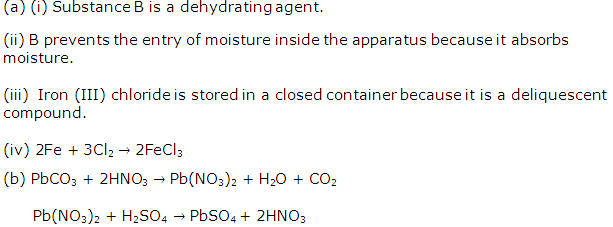
Study Of Acids, Bases and Salts Exercise 77
Solution 2010-1
(i) A
(ii) A
(iii) D
Solution 2010-2
![]()
Solution 2010-3
(i) ![]()
(ii) ![]()
(iii) ![]()
(iv) ![]()
![]()
Solution 2010-4
(i) C = Dilute sulphuric acid
(ii) A = Sodium hydroxide
(iii) B = Weak acid
Solution 2011-1
When crystals of washing soda are exposed to air, it loses its water of crystallisation and the phenomenon is known as efflorescence.
Solution 2011-2
(i) (B) Neutralisation
(ii) (E) Direct synthesis
(iii) (D) Double decomposition
(iv) (A) Simple displacement
(v) (C) Decomposition by acid
Solution 2013-1
(i) Hydronium
(ii) Hydroxide
(iii) Salt
(iv) Water
(v) Hydrogen
Study Of Acids, Bases and Salts Exercise 78
Solution 2016-1
(a) When washing soda (Na2CO3.10H2O) is exposed to air, it loses 9 molecules of water to form a monohydrate.
![]()
(b) It absorbs moisture from the atmosphere and becomes moist and ultimately dissolves in the absorbed water, forming a saturated solution.
Solution 2016-2
|
Column I |
Column II |
|
(i) P(NO3)2 from PbO (ii) MgCl2 from Mg (iii) FeCl3 from Fe (iv) NaNO3 from NaOH (v) ZnCO3 from ZnSO4 |
(A) Precipitation (F) Simple displacement (B) Combination (G) Neutralisation (H) Titration |
Solution 2017-1
(a) When a metallic oxide is dissolved in water, the solution formed has a high concentration of OH⁻ ions.
Solution 2017-2
(b) (c) An alkali