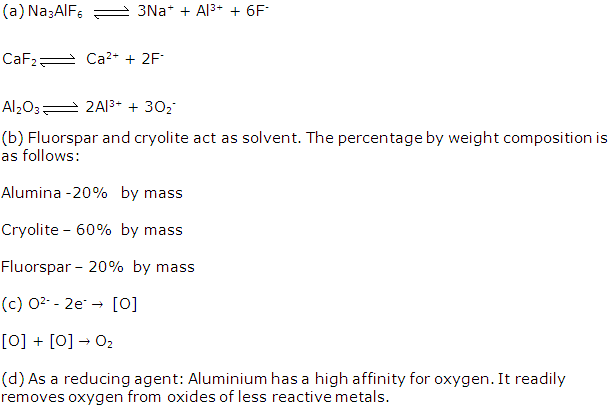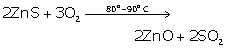Class 10 FRANK Solutions Chemistry Chapter 7 - Metallurgy
Revise your Chemistry lessons with our Frank Solutions for ICSE Class 10 Chemistry Chapter 7 Metallurgy. Study the definition of chapter-related terms such as flux, ore, slag, alloy, charge etc. Find out how an ore gets concentrated during the froth flotation process. The textbook solutions will also help you learn the concept of refining of metals along with the common refining methods.
Practise TopperLearning’s ICSE Class 10 Chemistry Frank Solutions to understand how to write balanced equations for oxides of carbon, reduction of iron and so on. In addition, use our concept videos, Selina Solutions, online practice tests and other Chemistry resources for board exam preparation.
Metallurgy Exercise 179
Solution 1
(i) Gold and Platinum.
(ii) Charge.
(iii) Gangue.
(iv) Flux.
(v) Calcination.
(vi) Roasting.
(vii) Iron pyrites.
(viii) Bauxite.
(ix) Cryolite, aluminium fluoride, Calcium fluoride.
(x) Cathode: inner lining of gas-carbon of the electrolytic cell.
Anode: Thick carbon rods dipping into the fused electrolytes.
(xi) Thermite welding.
(xii) Copper and silver.
(xiii) Aluminium, Iron.
(xiv) platinum and gold
(xv) sodium and potassium
Solution 2
(ii) Nitric acid can be stored in aluminium containers because it do not attack aluminium. It renders aluminium passive due to the formation of an oxide film on surface of aluminum.
(iii) Aluminium oxide cannot be reduced by carbon because it is comparatively high in electrochemical series hence more reactive than carbon.
(iv) A neutral gas other than oxygen is formed at the anode during electrolysis of fused alumina because the oxygen gas formed at the anode oxidizes the carbon of the anode to carbon dioxide.
(v) Extraction of aluminium was very difficult in the beginning because it was very expensive.
(vi) Carbon anodes are used in the electrolytic extraction of aluminium because carbon in the form of graphite is a good conductor of electricity.
(vii) Galvanized metal ions should not be used for storing food as food acids may react with the zinc coating and cause food poisoning.
Solution 3
(ii) Ore: Those minerals from which a metal can be extracted profitably are called ores.
(iii) Gangue: The rocky impurities like (SiO2) present in an ore are called gangue.
(iv) Charge: The mixture of materials fed into a furnace to extract a metal is called charge.
(v) Flux: The substance added to get rid of gangue in the extraction of metal is called flux.
(vi) Slag: The product obtained by the combination of gangue with flux is called slag.
Metallurgy Exercise 180
Solution 4
Solution 5
(b) Silver.
(c) Zinc.
(d) Aluminium.
Solution 6
Hence "All ores are minerals but all minerals are not ores".
Solution 7
(b) Zinc: Zinc blende (ZnS) and Calamine (ZnCO3).
(c) Aluminium:Bauxite (Al2O3) and Cryolite (AlF3.3NaF).
Solution 8
Solution 9
Refining of metals: It is the further purification of metals obtained by reduction process to remove all the impurities.
Depending upon the nature of metal, nature of impurities and purpose for which metal is to be used. The three methods used for refining are:
Liquation.
Distillation.
Electrolytic refining.
Solution 10

Solution 11

Solution 12
Solution 13
Solution 14

Solution 15
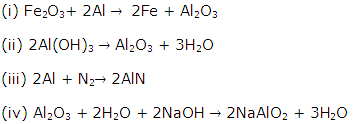
Solution 16
An alloy is a homogeneous mixture of two or more metals fused together and then solidified.
Alloys are made because they have many salient features:
Tensile strength.
Strength.
Electrical hardness.
Solution 17
1. Alloys are stronger and harder than the metals of which they are made.
2. Alloys are more resistant to corrosion.
Solution 18
Amalgam: A mixture or an alloy of mercury with a number of metals or non-metals is known as amalgams. An amalgam may be liquid such as Na/Hg or a solid like Zn/Hg.
Iron does not form amalgam.
Dental amalgam which is a mixture of mercury with a silver tin alloy is used for dental fillings.
Solution 19
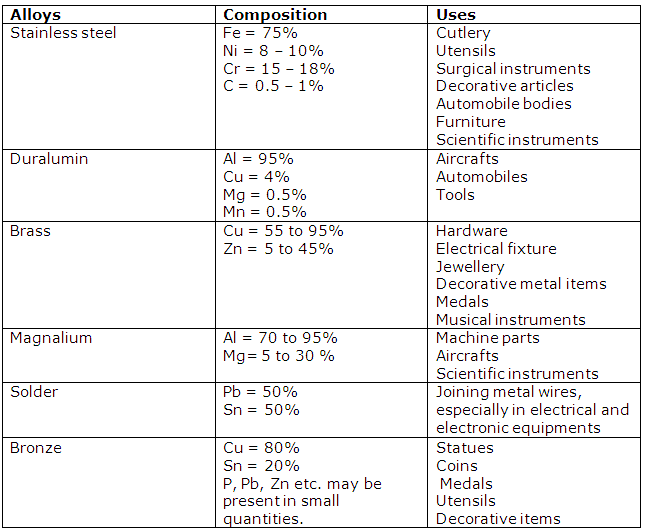
Solution 20
(ii) Stainless steel is more useful than steel as it is harder, has high tensile strength, more lustre, more resistance to corrosion and many chemicals.
(iii) Aluminium is extensively used for making aircraft parts because of features like high tensile strength, corrosion resistance light but hard and tough.
(iv) Cold water has no action on aluminium while burning aluminium decomposes steam.
Solution 21
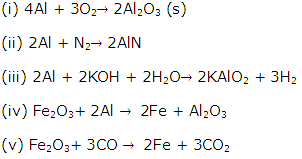
Solution 22
(i) Zinc oxide
![]()
(ii) Aluminium
![]()
(iii) Mercury
![]()
Metallurgy Exercise 181
Solution 23
(i) An aqueous solution of sodium chloride is not used for electrolytic reduction of sodium metal because if we electrolyse an aqueous solution of sodium chloride, then as soon as sodium metal is produced at the cathode, it will react with water to form sodium hydroxide. So electrolysis of aqueous sodium chloride solution will produce sodium hydroxide and not sodium metal.
(ii) Reducing agents for the reaction of a metal oxide: Aluminium, carbon monoxide and hydrogen
Solution 1991-1
(b) iron which cannot be easily acted upon by acidsis called as passive iron. Galvanized iron is called passive iron since coating of zinc protects the iron from corrosion as zinc is more electropositive and so would be attacked first.
Solution 1991-2
Solution 1992-1
Solution 1992-2
(a). Cryolite is Na3AlF6 and its chemical name is Sodium aluminium fluoride.
(b). Cryolite is used in the electrolysis of alumina. The function of cryolite is to
(i) Reduce melting point of alumina
(ii) Make molten alumina a good conductor of electricity
Solution 1992-3
(b)Iodine
(c) Bromine
(d) Carbon in the form of graphite
Solution 1993-1
Metallurgy Exercise 182
Solution 1993-2
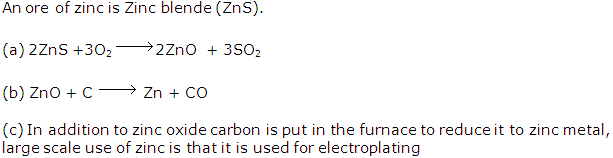
Solution 1994-1
Solution 1994-2
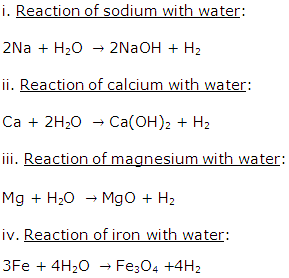
Solution 1995-1

Solution 1995-2
Solution 1996-1

Solution 1996-2

Solution 1996-3
An alloy is a homogeneous mixture of two or more metals fused together and then solidified.
(a) The special property of duralumin is:
Light but hard
Resistant to corrosion
Ductile
(b) Type metal = Hard
Solution 1997-1
Solution 1997-2
Ore Those minerals from which a metal can be extracted profitably are called ores. For example bauxite ore is used to extract aluminium metal, hematite ore is used to extract iron metal.
Solution 1998-1
(b) non-malleable.
(c) form negative ions.
(d) basic oxides.
Metallurgy Exercise 183
Solution 1998-2
(a) Mercury.
(b) Graphite.
Solution 1998-3
Solution 1999-1
Solution 1999-2
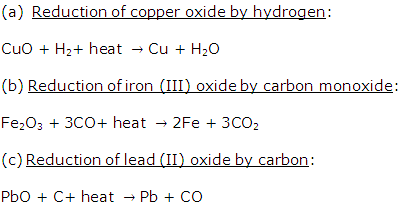
Solution 1999-3
(b) Metal would be obtained at cathode.
(c) Graphite, like metals is a good conductor of electricity.
Solution 2000-1
(b) Red
(c) Hydrogen
(d) acidic, acidic
(e) graphite.
Solution 2001-1
(b) Iron
(c) Zinc
(d) Magnesium
Solution 2001-2
Solution 2002-1
Metallurgy Exercise 184
Solution 2002-2
Solution 2003-1
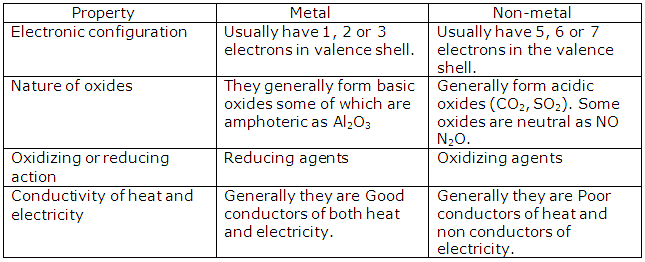
Solution 2004-1
Solution 2004-2
Solution 2004-3
Solution 2004-4
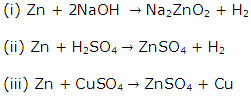
Metallurgy Exercise 185
Solution 2004-5
Galvanization.
Solution 2004-6
Solution 2005-1
(ii) A, C E
(b) (i) Sodium hydroxide solution
(ii) Cryolite
(C) Na3AlF6
Solution 2005-2
(b) For brass: Copper and zinc.
Solution 2006-1
(b) Cryolite.
(c) Roasting.
(d) Calcium silicate.
(e) Zone of heat absorption.
Solution 2007-1
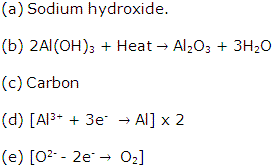
Metallurgy Exercise 186
Solution 2009-1
(b) Mercury as it is a liquid metal while the rest aresolid.
Solution 2009-2
(b) Bauxite is the chief ore of aluminium.
Solution 2009-3
(b) Molten fluorides of Al, Na and Ba.
(c) Graphite rods.
Solution 2009-4
Solution 2008-1
(b)
Metallurgy Exercise 187
Solution 2010-1
(a) Metals are good conductors of electricity.
True
(b) Metals are malleable and ductile.
True
(c) Metals form non-polar covalent compounds.
False (Metals form ionic compounds.)
(d) Metals have 1 or 2 or 3 electrons in their valence shell.
True
Solution 2011-1
(i) Duralumin
Al (95%)
Cu (4%)
Mg (0.5%)
Mn (0.5%)
(ii) Brass
Cu (60-70%)
Zn (30-40%)
(iii) Stainless steel
Fe (73%)
Cr (18%)
Ni (8%)
C (1%)
Solution 2011-2
Mercury
Solution 2011-3
(i) A metal which is found abundantly in the Earth's crust is aluminium.
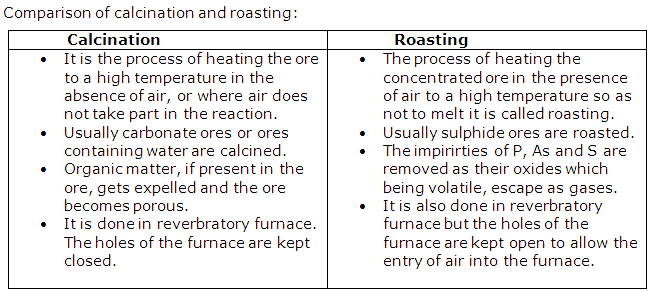
(ii) Differences between calcination and roasting:
|
Calcination |
Roasting |
|
1. The ore is heated in the absence of air. |
1. The ore is heated in excess of air. |
|
2. Moisture and organic impurities are removed, and the ore becomes porous and more reactive. |
2. Volatile impurities are removed as oxides (SO2, P2O5, As2O3), and the ore becomes porous and more reactive. |
|
3. Carbonate and hydrated ores are calcined, and CO2 or water vapour is given off. ZnCO3 → ZnO + CO2 |
3. Sulphide ores are roasted, so SO2 is given off.
|
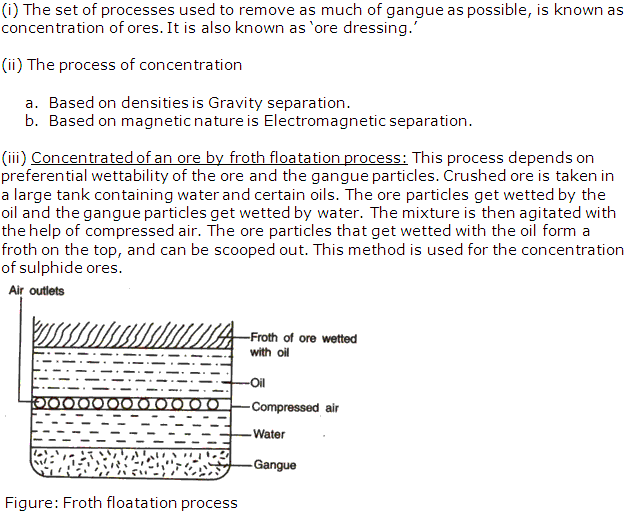
(iii) Froth flotation process is used for the enrichment of sulphide ore.
(iv) Ore of iron
|
Name |
Chemical name |
Formula |
|
Red haematite |
Anhydrous ferric oxide |
Fe2O3 |
|
Brown haematite |
Hydrated ferric oxide |
2Fe2O3.3H2O |
Ores of aluminium
|
Name |
Chemical name |
Formula |
|
Bauxite |
Hydrated aluminium oxide |
Al3O32H2O |
|
Cryolite |
Sodium aluminium oxide |
Na3AlF6 |
(v) Constituents of the electrolyte for the extraction of aluminium are pure alumina (Al2O3) cryolite (Na3AlF6) and fluorspar (CaF₂).
Solution 2013-1
(i) Y is the metallic element.
(ii) Metal atoms tend to have a maximum of 3 electrons in the outermost energy level.
(iii) Non-metallic elements tend to form acidic oxides, while metals tend to form basic oxides.
(iv) Non-metallic elements tend to be poor conductors of heat and electricity.
(v) Metals tend to lose electrons and act as reducing agents in their reactions with elements and compounds.
Metallurgy Exercise 188
Solution 2013-2
Bronze
Solution 2013-3
Extraction of aluminium:
(i) Cryolite (Na3AlF6) is added along with alumina and fluorspar. It lowers the fusion temperature from 2050°C to 950°C and enhances conductivity.
(ii) Al3+ + 3e-→ Al
(iii) It is necessary to renew the anode periodically as it gets oxidised by the oxygen evolved at the anode.
Solution 2014-1
(i) Main components of brass are copper and zinc.
(ii) Main components of duralumin are aluminium, magnesium, copper and manganese.
(iii) Main components of bronze are copper, zinc and tin.
Solution 2014-2
(i) Malleability is the property possessed by metals by which they can be beaten into sheets.
(ii) Cryolite is added to lower the fusion temperature of an electrolytic bath in the extraction of aluminium.
(iii) Zinc blende (sphalerite) is the ore of zinc containing its sulphide.
Solution 2014-3
(a) The main ore used for the extraction of iron is
haematite.
Solution 2014-4
(b) Heating an ore in a limited supply of air or in the absence of air at a temperature just below its melting point is known as calcination.
Solution 2014-5
(a) It is a strong reducing agent.
Solution 2015-1
(c) Solder
Solution 2015-2
In the extraction of aluminium, the given compounds play the following roles:
(a) Cryolite: It lowers the fusion temperature from 2050°C to 950°C and enhances conductivity.
(b) Sodium hydroxide:
Two roles are played by sodium hydroxide in the extraction of aluminium.
First, finely grinded bauxite (ore of aluminium) is heated under pressure with conc. caustic soda solution (NaOH solution) for 2-8 hours at 140°C to 150°C to produce sodium aluminate. The chemical equation is as follows:
Al2O3.2H2O + 2NaOH → 2NaAlO2+ 3H2O
Second, on diluting sodium aluminate with water and
cooling to 50°C, sodium aluminate is hydrolysed to give
aluminium hydroxide as a precipitate. Here, the impurities
dissolve in sodium hydroxide.
(c) Graphite: Thick rods of graphite are suspended in the fused electrolyte. They act as an anode where oxygen gas is discharged.
Solution 2015-3
(i) In the electrolysis of alumina using the Hall-Héroult process, the electrolyte is covered with powdered coke as it
a. reduces heat loss by radiation
b. prevents the burning of the anode
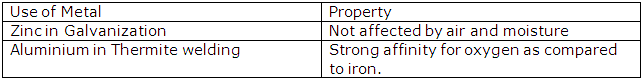
(ii) Iron sheets are coated with zinc during galvanisation to prevent them from rusting.
Metallurgy Exercise 189
Solution 2016-1
(i) Conc. caustic soda
(ii) ![]()
(iii) Cryolite
(iv) At the cathode: Al3++ 3e-→ Al
(v) The anode has to be replaced from time to time as it gets oxidised by oxygen evolved at the anode.
Solution 2016-2
Copper and tin
Solution 2017-1
(i) Electroplating
(ii) Solder
(iii) Zinc blende
(iv) PbO or CuO
Solution 2017-2
(i) Components of electrolyte: Cryolite and fluorspar
Role played by each electrolyte is given below:
a. Cryolite lowers the fusion temperature from 2050°C to 950°C and enhances conductivity.
b. Fluorspar and cryolite act as a solvent for the electrolytic mixture and increase conductivity.
(ii) Powdered coke is sprinkled over the surface of the electrolytic mixture for the following reasons:
a. To reduce heat loss by radiation
b. To prevent burning of the anode
Solution 2017-3
(i) Cu
(ii) Pb
(iii) Al
Solution 2017-4
(i) The process of crushing ores into a fine powder in big crushers and ball mills is known as pulverisation.
(ii) Heating of the ore in the absence of air to a high temperature that is high but insufficient to melt the ore is known as calcination.