Class 10 FRANK Solutions Chemistry Chapter 6 - Electrolysis
Improve your Chemistry skills with our Frank Solutions for ICSE Class 10 Chemistry Chapter 6 Electrolysis. Go through the definitions of cation, electro metallurgy, anion, electrolysis, electrolyte and non-electrolyte. Revise the steps to explain the purification of impure copper using the electrolysis method. Also, revisit the various applications of electrolysis.
In addition, relearn equations for reactions which occur during the electrolysis of lead bromide with our ICSE Class 10 Chemistry Frank Solutions. To learn further about electrolysis, practise TopperLearning’s Selina Solutions, sample question paper solutions etc.
Electrolysis Exercise 145
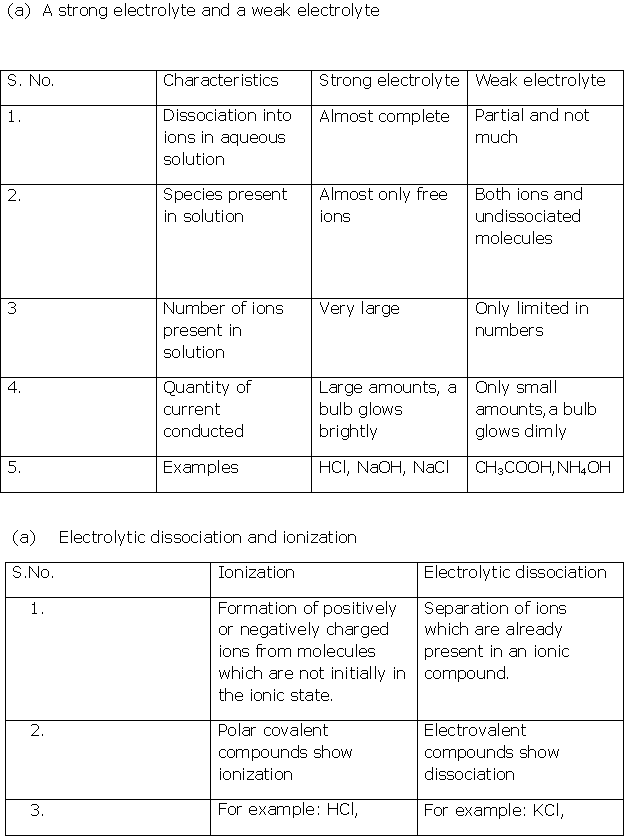
Solution 1

Solution 2

Solution 3
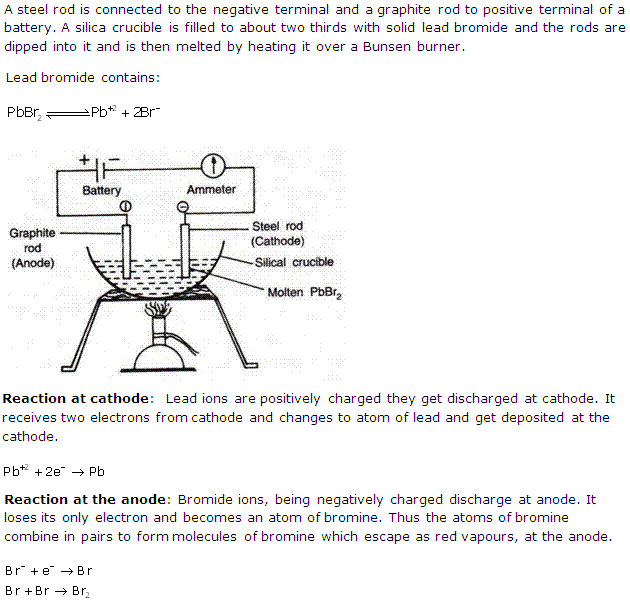
Solution 4
Solution 5
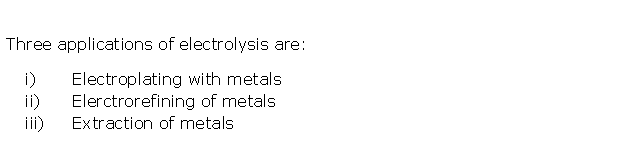
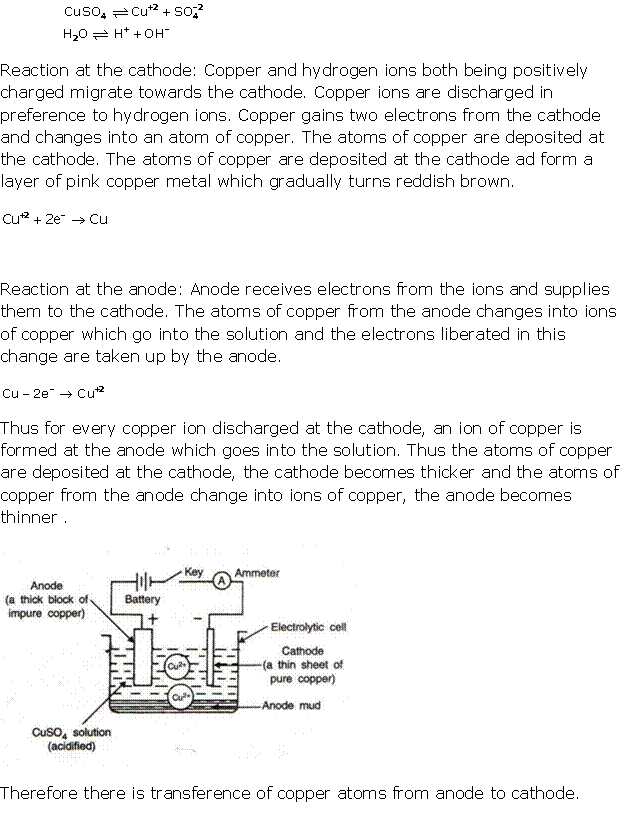
Solution 6

Solution 7

Solution 8
Solution 9

Electrolysis Exercise 146
Solution 10
Solution 11
Solution 12
Solution 13

Solution 14

Solution 15

Solution 16

Solution 17
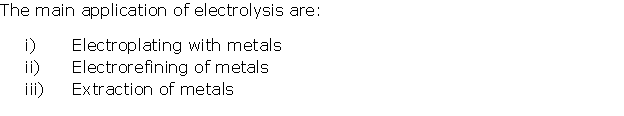
Solution 18

Solution 19

Solution 20

Solution 21
(i) CuSO4
(ii) Au3+
(iii) OH⁻
(iv) Graphite rod
(v) Hydrogen and oxygen
Electrolysis Exercise 147
Solution 1994-1
(b) The ions of the metal which is to be electroplated must be present in the electrolyte.
(c) The metal to be plated on the article must be made anode. It needs to be periodically replaced.
Solution 1994-2
Solution 1995-1

Solution 1995-2
Solution 1995-3
Solution 1996-1
(i) Ions only:- HCl
(ii) Molecules only:- Petrol
(iii) Both ions and molecules:- CH3COOH
Solution 1996-2
(b) Non- electrolyte are substances which do not conduct electricity in fused or aqueous state. They contain only molecules and do not ionize. For example: petrol, alcohol.
(c) If the electrolyte is described as 'strong electrolyte' it means it completely dissociates into its constituting ions in aqueous solution.
Solution 1996-3
(b) When platinum rods are used as electrodes, then x the blue colour of copper sulphate solution fades and sulphuric acid is formed. This is because oxygen is liberated at anode and copper metal is deposited at cathode
(c) Practical application of electrolysis of copper sulphate solution: This is the basis for purification of copper.
Other metals like Zinc, Nickel, Silver .Lead can also be purified.
Electrolysis Exercise 148
Solution 1997-1
Solution 1997-2
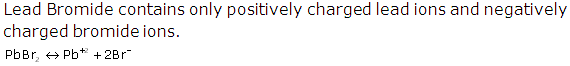
Solution 1997-3

Solution 1998-1
(b) Nickel
(c) cathode
(d) anode
(e) Cations
Solution 1999-1
Solution 1999-2
Solution 2000-1
(b) Weak electrolyte - Acetic acid, Ammonium hydroxide
(c) Non-electrolytes - Carbon tetrachloride
Solution 2002-1
(ii) will not
Solution 2002-2
(b) Cathode, Anode
Solution 2002-3
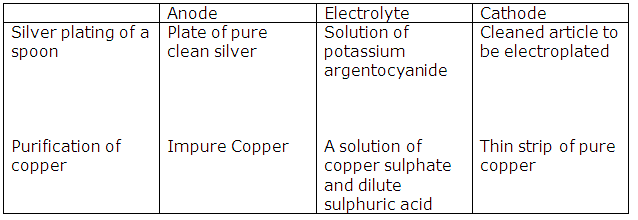
Solution 2003-1
Electrolysis Exercise 149
Solution 2004-1
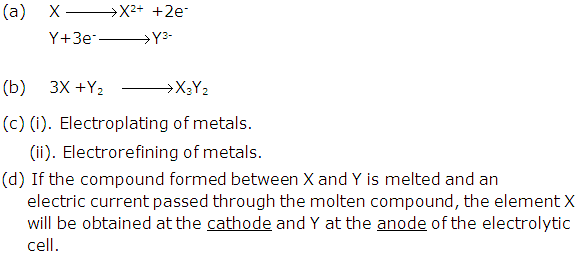
Solution 2004-2
(b) Non ionized molecules; H+ and X- particles will be present in dilute solution.
(c) Loss, Gain
(d) The ions of the metal which is to be electroplated on the article must be present in a solution.
(e) Redox reaction is one in which oxidation and reduction occurs simultaneously.
Similarly in case of electrolysis:
At cathode: The cations gain electron and become neutral. As the electrons are gained the ion is said to be reduced.
At anode: The anions lose electron to form neutral atoms. As the electrons are lost the ion is said to be oxidized.
Hence in electrolysis also the oxidation and reduction occurs hence it is an example of Redox reaction.
Solution 2005-1
(ii) Hydrogen is released at the cathode when acidulated water is electrolyzed.
(iii) In sodium chloride, Na+ and Cl- ions are not free to carry the electric current.
(iv) (a) Reduced
(b) Higher
Solution 2006-1
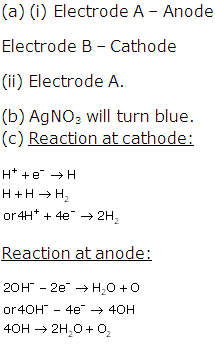
Solution 2006-2

Solution 2007-1
(ii) Carbon tetrachloride- Non-electrolyte
(iii) An aluminium wire- Metallic conductor
(iv) A solution containing solvent molecules, solute molecules and ions formed by the dissociation of solute molecules- weak electrolyte
(v) A sugar solution with sugar molecules and water molecules- Non-electrolyte
Electrolysis Exercise 151
Solution 2009-2
Mg(OH)2 as it is basic while rest are amphoteric.
Solution 2009-3
Solution 2009-4
(b) Nickel ions.
Solution 2010-1
(i) Lead (II) bromide
(ii) Oxidation
Solution 2010-2
(i) Aqueous solution of nickel sulphate with few drops of dil. sulphuric acid
(ii) Article (e.g. key chain)
(iii) Pure nickel
(iv) Ni2+ + 2e-→ Ni
(v) Ni → Ni2+ + 2e-
Solution 2010-3
Cell A contains sodium chloride solution which is a strong
electrolyte and contains only ions. So, it conducts electricity
and the bulb glows brightly.
Cell B contains both ions and molecules. So, there are few
ions to conduct electricity and the bulb glows dimly.
Cell C contains sugar solution which is a non-electrolyte and
does not contain ions. So, it is a bad conductor of electricity
and the bulb does not glow.
Solution 2011-1
Dilute sulphuric acid catalyses dissociation, so electrolysis of acidified water is considered an example of catalysis.
Solution 2011-2
(i) In covalent compounds, the bond is formed due to the sharing of electrons.
(ii) Electro covalent compounds have a high boiling point.
(iii) A molecule of nitrogen contains a triple bond.
Solution 2011-3
|
Copper sulphate solution |
Copper metal |
|
Conduction of electricity is due to the flow of ions. |
Conduction of electricity is due to the flow of electrons. |
|
It is an aqueous solution of an ionic compound. |
It is a metal in the solid state. |
|
It undergoes a chemical change. |
It remains unchanged chemically. |
Solution 2011-4
(i) Red shiny metal is deposited at the cathode.
(ii) The colour of the electrolytes changes gradually from blue to colourless.
(iii) At the cathode:
Cu2+ + 2e- → Cu
Reaction at the anode:
OH- → OH + e-
4OH → 2H2O + O2
Electrolysis Exercise 150
Solution 2007-2

Solution 2008-1
Solution 2008-2

Solution 2008-3
(b) At electrode A.
(c) Two compounds in the electrolyte are Al2O3 and Na3AlF6
(d) As at electrode B the oxygen is liberated during the process. The oxygen liberated oxidizes the carbon anode producing CO and CO2.Thus electrode B is to be replaced continuously.
Solution 2009-1

Electrolysis Exercise 152
Solution 2013-1
(i) The right electrode is the anode and oxidising electrode Cu → Cu2+ + 2e- losing electrode.
(ii) Reaction at the anode: Cu → Cu2+ + 2e-
Reaction at the cathode: Cu2+ + 2e- → Cu
(iii) The anode dissolves and anode mud containing precious metal is recovered.
Solution 2013-2
(i) Liquid carbon tetrachloride
Solution 2013-3
Dark reddish brown fumes of bromine evolve at the anode and greyish white metal lead is formed on the cathode.
Solution 2014-1
(iii) A silver grey deposit at the cathode and reddish brown fumes at the anode.
Solution 2014-2
(iii) Sodium argentocyanide solution
Solution 2014-3
(i) Electrovalent or ionic compounds
(ii) One electron
(iii) Since it has valency 1, M belongs to Group 1.
(iv) At the cathode: M+ + 1e- → M
(v) At the anode: Oxygen gas
Solution 2015-1
(i) Zinc is lower in the reactivity series, so it is comparatively less reactive. Hence, it is reduced by using carbon monoxide. But aluminium is very reactive; hence, it cannot be reduced by using a reducing agent and it can be reduced only by electrolytic reduction.
(ii) Carbon tetrachloride is a liquid and does not conduct electricity because it is a covalent compound and there are no free ions present and it contains only molecules.
(iii) In electrolysis of molten lead bromide, reactive bromine is liberated at the anode. As bromine is very reactive, an inert electrode like graphite is preferred in the electrolysis of molten lead bromide.
(iv) Acetic acid is a weak acid and has fewer ions, so conductivity is less, whereas dilute sulphuric acid is a strong acid and has more ions, and therefore, its electrical conductivity is more.
(v) During electrolysis of lead bromide, there is loss of electrons at the anode by bromine and gain of electrons at the cathode by lead. Thus, oxidation and reduction occur side by side. So, it is a redox reaction.
PbBr2 ⇌ Pb+2 + 2Br-
Solution 2015-2
|
Strong Electrolytes |
Weak Electrolytes |
|
Electrolytes which allow a large amount of electricity to flow through them. |
Electrolytes which allow small amounts of electricity to flow through them. |
|
These are good conductors of electricity. |
These are poor conductors of electricity. |
|
These almost completely dissociate in the fused or aqueous solution state. |
These are partially dissociated in the fused or aqueous solution state. |
|
These solutions contain only free mobile ions. |
These solutions contain ions as well as molecules. |
Electrolysis Exercise 153
Solution 2015-3
|
|
Anode |
Electrolyte |
|
Purification of copper |
Impure copper |
Solution of copper sulphate and dilute sulphuric acid |
(i)
(ii) Ag - e- → Ag+
Cu - e- → Cu2+
Cl⁻ ‒ e- → Cl
Cl + Cl → Cl2
Solution 2016-1
(i) Electrostatic forces of attraction between ions in the solid state are very strong. These forces weaken in the fused state or in the solution state. Hence, ions become mobile.
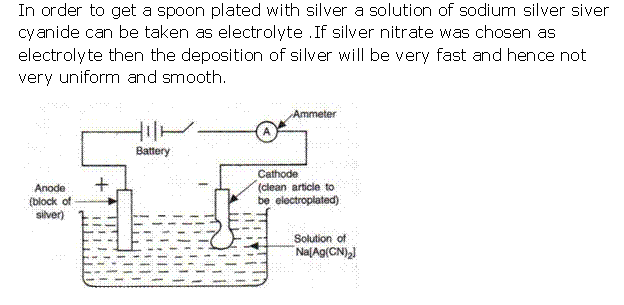
(ii) If silver nitrate solution is used directly instead of double cyanide of silver and sodium, the deposition of silver will be very fast and hence not very smooth and uniform.
(iii) Copper has no mobile electrons in the solid state and an electrolyte should dissociate into oppositely charged ions to conduct electricity.
Hence, copper is a non-electrolyte.
Solution 2016-2
(i) Oxygen is the product formed at the anode.
(ii) Ag+ and Na+
Solution 2016-3
(i) Electrodes:
Cathode: Copper
Anode: Platinum
Reaction at the cathode: Cu2+ + 2e-→ Cu
Reaction at the anode: 4OH- - 4e-→ 4OH
2OH + 2OH →2H2O + O2
(ii) The cathode and anode are both made of graphite plates.
Reaction at the cathode: Pb2+ + 2e-→Pb
Reaction at the anode: Br- - e-→Br
Br + Br →Br2
Solution 2017-1
(i) The electrolyte used for electroplating an article with silver: Sodium argentocyanide or potassium argentocyanide
(ii) The particles present in a liquid such as kerosene that is a non-electrolyte: Molecules
Solution 2017-2
(i) Observations:
Anode: Dark reddish brown fumes of bromine evolve at the anode.
Cathode: Greyish white metal lead is formed on the cathode.
(ii) Observations:
Anode: Nothing gets deposited on the anode because the copper anode dissolves during the reaction as Cu2+ ions are formed.
Cathode: Reddish brown Cu is deposited.
Solution 2017-3
(a) OH-
(b) Ag+

