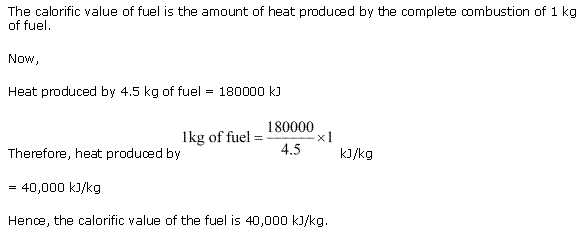Class 8 NCERT Solutions Science Chapter 4 - Combustion and Flame
Combustion and Flame Exercise 51
Solution 1
Combustion is a process of reaction of a substance with oxygen. There are certain conditions required for combustion to take place. They are:
(i) Presence of a fuel
(ii) Air (or oxygen)
(iii) Ignition temperature (minimum temperature at which a substance catches fire)
Concept insight: below ignition temperature combustion cannot takes place
Solution 2
(a) pollution
(b) kerosene
(c) ignition temperature
(d) water
Solution 3
As the amount of unburnt carbon particles produced is very less in comparison to petrol and amount of harmful gases produced is also very less ,so CNG is comparatively a cleaner fuel than petrol.
Solution 4
Solution 5
(a) Water is a good conductor of electricity. If it is used for controlling a fire involving electrical equipments, then the person dousing the fire might get an electric shock. Also, water can damage electrical equipments.
(b) LPG is a better domestic fuel because being a gaseous fuel it does not produce smoke and un-burnt carbon particles, which cause respiratory problems.
(c) A piece of paper wrapped around aluminium pipe does not catch fire easily. This is because aluminium, being a metal, is a good conductor of heat. Therefore, heat is transferred from the paper to the metal and the paper does not attain its ignition temperature.
Solution 6
Solution 7
The calorific value of a fuel is expressed in kilojoules per kilogram (kJ/kg).
Solution 8
CO2 is a non-combustible gas and it is also non supporter of combustion. It extinguishes fire in two ways:
(i) Since it is heavier than oxygen, it covers the fire like a blanket and cuts off the contact between oxygen and fuel.
(ii) In cylinders, CO2 is kept in the liquid form. When released, it expands enormously and cools down. This brings down the temperature of the fuel, which helps in controlling the fire.
Solution 9
Green leaves have a lot of moisture in them. This moisture does not allow them to catch fire easily. However, dry leaves have no moisture in them. Therefore, they catch fire easily.
Solution 10
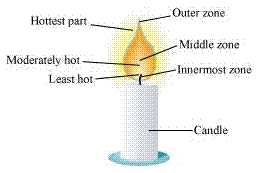
Goldsmiths use the outermost part/zone of the flame to melt gold and silver. This is because the outermost zone of the flame undergoes complete combustion and is the hottest part of the flame.
Solution 11
Solution 12
Yes, rusting is a kind of slow combustion because in this iron combines with oxygen giving out heat.
Solution 13
The water in the Ramesh's beaker will heat up in a shorter time. This is because the outermost zone of a flame is the hottest zone, while the yellow zone (in which Abida had kept the beaker) is less hot.