Class 9 SELINA Solutions Chemistry Chapter 3: Water
Water Exercise Ex. 3(A)
Solution 1
In the free state, water occurs in the solid, liquid and gaseous states.
- Solid state: A large amount of fresh water is found in the form of snow or ice.
- Liquid state: Most of the water present in oceans and found in streams, rivers, lakes, ponds and springs on land is water in the liquid state.
- Gaseous state: Water vapour present in the air is in the gaseous state. Water vapour condenses in the sky to form clouds. Mist and fog are also examples of water in the gaseous form.
Solution 2
Water is considered a compound because it is made of two elements hydrogen and oxygen combined in the ratio 1:8 by mass.
Mass ratio of elements H2O
H : O, 2 × 1 : 16 × 1 = 1 : 8
(Atomic mass of H = 1, O = 16)
Components of water cannot be separated by physical methods but can be separated by electrolysis of water.
Solution 3
a) The temperature in Mumbai and Chennai do not fall as low as in Delhi because these cities are situated in the coastal areas. Due to high specific heat capacity, the presence of a large amount of water is able to modify the climate of the nearby land areas making them warmer in winter and cooler in summer. So, the temperature does not fall as low as it does in Delhi.
b) Our body is almost 65% of water, and it has the property of specific heat. Due to high specific heat capacity, the presence of a large amount of water is able to modify the climate of the body and control the temperature of our body, which is warm in winter and cool in summer.
Solution 4
Water dissolves many substances forming an aqueous solution. It can dissolve solids, liquids and gases. When a solid dissolves in water, the solid is the solute, the water is the solvent and the resultant liquid is the solution. So, it is said that water is a universal solvent. In other words, water can dissolve nearly every substance.
Solution 5
The sudden release of the latent heat of condensation causes the violence associated with torrential rain.
Solution 6
- Due to the high specific heat capacity, the presence of a large amount of water is able to modify the climate.
- The property of anomalous expansion of water enables marine life to exist in the colder regions of the world, because even when water freezes on the top, it is still liquid below the ice layer, as the density of water is greater than that of ice.
- The boiling point of water increases due to the presence of dissolved impurities.
The freezing point of water decreases due to the presence of dissolved impurities.
Solution 7
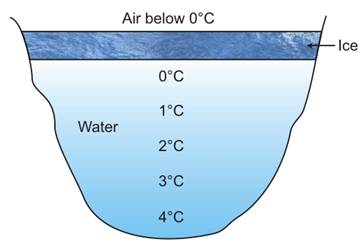
Water has an unusual physical property. When cooled, it first contracts in volume, as do other liquids, but at 4°C (maximum density), it starts expanding, and continues to do so till the temperature reaches 0°C, the point at which it freezes into ice.
The property of anomalous expansion of water enables marine life to exist in the colder regions of the world, because even when the water freezes on the surface, it is still liquid below the ice layer.
|
Formation of ice on the water surface of a pond |
Solution 8
|
Properties of water are different from the properties of elements from which it is formed. |
||
|
Property |
Water |
Elements - Oxygen and Hydrogen |
|
Nature |
It is a clear, colourless, odourless, tasteless and transparent liquid. |
These are colourless, odourless, tasteless and non-poisonous gases. |
|
Solubility |
It can dissolve many substances and is called a universal solvent. |
Oxygen and hydrogen are slightly soluble in water. |
|
Density |
Pure water has maximum density at 4°C. |
Oxygen is heavier than air, and hydrogen is the lightest of all the known gases. |
Solution 9
The property of anomalous expansion of water enables aquatic life to exist because water freezes on the surface of the water body, but it is still liquid below the ice layer.
Solution 10
When tap water is boiled and evaporated:
Observations:
• A number of concentric rings of solid matter are seen on the watch glass after evaporation of tap water.
Conclusion:
• Tap water contains dissolved salts, minerals and impurities.
Solution 11
Importance of dissolved salts in water:
- Dissolved salts provide specific taste to water.
- Dissolved salts act as micronutrients for the growth and development of living beings.
Solution 12
They add taste to water for drinking purposes.
Solution 13
Oxygen is more soluble in water than nitrogen. Air dissolved in water contains a higher percentage of oxygen (30-35%). Oxygen is only 21% in ordinary air. In this way, air dissolved in water is different from ordinary air.
Solution 14
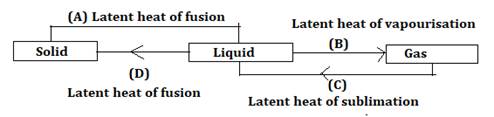
(B) - Latent heat of vapourisation
(C) - Latent heat of sublimation
(D) - latent heat of fusion
Solution 15
- Boiled water tastes flat because it does not contain dissolved matter such as air, carbon dioxide and other minerals.
- Ice at 0°C gives more cooling effect than water at 0°C because at 0°C ice absorbs 336 J per gram of energy to melt to 0°C water.
- Burns caused by steam are more severe than burns caused by boiling water because of high specific latent heat of condensation. 2268 J/g of heat is released when 1 g of steam condenses to form 1 gm of water.
- Due to the high specific latent heat of solidification of water, rivers and lakes do not freeze easily.
- Air dissolved in water contains a higher percentage of oxygen because the solubility of oxygen in water is more than that of oxygen in air.
- If distilled water is kept in a sealed bottle for a long time, it etches the surface of glass because substances which are apparently insoluble in water actually dissolve in minute traces in water.
- Rain water does not leave concentric rings when boiled because rain water does not contain dissolved solids.
Water Exercise Ex. 3(B)
Solution 1
- Solution: A solution is a homogeneous mixture of two or more substances, the components of which cannot be seen separately.
- Solute: A solute is the substance which dissolves in a solvent to form a solution.
- Solvent: A solvent is the medium in which a solute dissolves.
|
Solution = Solute + Solvent |
Solution 2
Solubility of nitrates decreases with a fall in temperature. Thus, when a hot saturated solution of potassium nitrate cools, it forms crystals as it separates from the solution.
Solution 3
Three factors on which the solubility of a solid depend:
- Temperature
- Nature of the solid
- Nature of the solvent
Solution 4
- Take 100 g of distilled water in a beaker. Add to this one gram of copper sulphate crystals.
- Stir this mixture with the help of a glass rod and dissolve the copper sulphate crystals. Similarly, go on dissolving more copper sulphate (1 gram at a time) with constant and vigorous stirring. A stage is reached when no more copper sulphate dissolves. It is called a saturated solution at this temperature.
Solution 5
- Henry's law: At any given temperature, the mass of a gas dissolved in a fixed volume of a liquid or solution is directly proportional to the pressure on the surface of a liquid.
- Crystallisation: It is the process by which crystals of a substance separate out on cooling its hot saturated solution.
- In the laboratory, crystals may be obtained by the following methods:
- By cooling a hot saturated solution gently
- By cooling a fused mass
- By sublimation
- By slowly evaporating a saturated solution
Solution 6
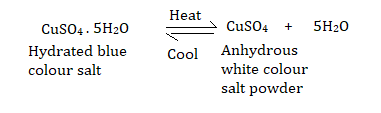
When copper (II) sulphate crystals are heated, drops of colourless liquid condense on the cooler parts of the test tube, leaving behind a residue that is anhydrous (without water) and amorphous (non-crystalline), i.e. with no definite shape or structure.
When blue crystals of hydrated copper sulphate are heated in a test tube, they turn into a white powder, which turns back into a blue solid when a few drops of water are added.
Solution 7
i. Washing soda crystals: Na2CO3.10H2O
ii. Blue vitriol: CuSO4.5 H2O
i. Table salt: NaCl
ii. Nitre: KNO3
- Sulphuric acid: H2SO4
- Quick lime: CaO
Solution 8
Solubility of potassium nitrate (KNO3) in water increases with an increase in temperature.
Solubility of calcium sulphate (CaSO4) in water decreases with an increase in temperature.
Solution 9
Solubility of NaCl at 40°C is 36.5 g means that 36.5 g of NaCl dissolves in 100 g of water at a temperature of 40°C.
Solution 10
- A solution in which more of a solute can be dissolved at a given temperature is an unsaturated solution.
- A solution in which no more solute can be dissolved at a given temperature is a saturated solution at that temperature.
- A solution in which some solute separates on cooling slightly is a super saturated solution.
Solution 11
With an increase in pressure, the solubility of a gas in water increases.
With an increase in temperature, the solubility of a gas in water decreases.
For example, the solubility of carbon dioxide in water under normal atmospheric pressure is low, but when the water surface is subjected to higher pressure, a lot more of CO2 gas gets dissolved in it.
Similarly, in case of soda water, on opening the bottle, the dissolved gas rapidly bubbles out because the pressure on the surface of the water suddenly decreases.
Solution 12
- Non-aqueous solution
- Deliquescence
- Hydrated substance
- Hygroscopy
- Efflorescence
- Dehydrating agent
Solution 13
- Water is an excellent liquid to use in cooling systems because of its high specific heat.
- A water-soluble solid disappears in a solution where the solvent is water, and water has the property of being clear and transparent. So, the solution is also clear and transparent.
- Lakes and rivers do not freeze suddenly in winters because of the high specific latent heat of solidification, i.e. the amount of heat released when 1 g of water solidifies to form 1 g of ice at 0°C. It is about 336 J/g or 80 cal/g.
- The component which dissolves in a solvent is known as a solute. So, it cannot be separated from a solution by filtration. However, filtration is used when the solute is insoluble in the solution.
- Fused CaCl2 or concentrated H2SO4 is deliquescent in nature, i.e. it absorbs moisture, and hence, these are used in desiccators as drying agents.
- Carbon dioxide is dissolved in soda water under pressure. On opening the bottle, the pressure on the surface of water suddenly decreases; therefore, the solubility of CO2 in water decreases and the gas rapidly bubbles out.
- Table salt becomes sticky on exposure during the rainy season, because it generally contains a small percentage of magnesium chloride and calcium chloride as impurities. These impurities absorb moisture from the monsoon air due to their deliquescent nature, and thus, table salt become sticky.
Solution 14
- Potassium nitrate
- Potassium chloride
- Sodium chloride
- Calcium sulphate
Solution 15
These are substances which can readily absorb moisture from other substances without chemically reacting with them.
Examples:
Phosphorous pentoxide (P2O5), quick lime (CaO)
Solution 16
|
Common Name |
Chemical Name |
Formula |
Acid, base or salt |
Efflorescent, hygroscopic or deliquescent substance |
|
Solid caustic potash |
Potassium hydroxide |
KOH |
Base |
Deliquescent substance |
|
Quick lime |
Calcium oxide |
CaO |
Base |
Hygroscopic substance |
|
Oil of vitriol |
Sulphuric acid |
H2SO4 |
Acid |
Hygroscopic substance |
|
Washing soda |
Hydrated sodium carbonate |
Na2CO3.10H2O |
Salt |
Efflorescent substance |
|
Solid caustic soda |
Sodium hydroxide |
NaOH |
Base |
Deliquescent substance |
|
Blue vitriol |
Copper sulphate |
CuSO4 |
Salt |
Efflorescent substance |
Solution 17
- Increase in mass: Iron and conc. sulphuric acid
- Decrease in mass: Sodium carbonate crystals
- No change in mass: Sodium chloride
Solution 18
salt becomes powdery anhydrous sodium sulphate when exposed to dry air.
![]()
Water Exercise Ex. 3(C)
Solution A. 1
Correct option: (ii) - Water
Sodium sulphate is soluble in water.
Solution A. 2
Correct option: (ii) - It is polar and has a high dielectric constant.
Solution A. 3
Correct option: (ii) - It is polar and has a high dielectric constant.
Solution A. 4
Correct option: (iii) - Alloys
Solution A. 5
Correct option: (iv) - Increase slightly
Solution A. 6
Correct option: (ii) - Hydrated sodium sulphate
Solution A. 7
Correct option: (iii) - Potassium permanganate
Solution A. 8
Correct option: (i) - It is deliquescent
Solution A. 9
Correct option: (iv) - Conc. sulphuric acid
Solution A. 10
Correct option: (ii) - FeCl3
Solution A. 11
Correct option: (iii) - Hydrated
Solution A. 12
Correct option: (ii) - Magnesium bicarbonate
Solution A. 13
Correct option: (ii) - Boiling
Solution B. 1
(a) The solute in sugar solution : sugar
The solvent in sugar solution : water
(b) The characteristic property which makes water the universal solvent. :
Polarity and high dielectric solvent
(c) A substance whose solubility shows an anomalous behavior. :
Na2SO4.10H2O (Glauber's salt)
(d) A substance whose solubility rapidly increases with the temperature.
Sodium nitrate, Potassium nitrate, Potassium bromide
Solution B. 2
The composition of water is 2 atoms of hydrogen with 1 atom of oxygen (H2O).
By number of atoms, they combine in the ratio 2:1.
Solution B. 3
- Hydrogen carbonates of calcium and magnesium
- Sulphates and chlorides of magnesium and calcium
Solution B. 4
Methods by which hydrous substances can be made anhydrous:
- By heating
- Exposure to dry air
Solution C. 1
Air dissolved in water is biologically very important.
• Oxygen dissolved in water is used by marine life like fish for respiration, and thus, marine life is sustained.
• Aquatic plants make use of dissolved carbon dioxide in photosynthesis to prepare food.
• Carbon dioxide dissolved in water reacts with calcium carbonate to form calcium bicarbonate.
Marine organisms such as oysters and snails extract calcium carbonate from calcium bicarbonate to build their shells.
Solution C. 2
Solubility of sodium nitrate decreases with a fall in temperature. Thus, when a hot saturated solution of sodium nitrate cools, it forms crystals as it separates from the solution.
Solution C. 3
- Water is said to be soft when the water containing sodium salts easily gives lather with soap.
- Water is said to be hard when it does not readily form lather with soap.
- Water which contains only hydrogen carbonates of calcium and magnesium is called temporary hard water.
- Water containing sulphates and chlorides of magnesium and calcium is called permanent hard water.
Solution C. 4
- The presence of hydrogen carbonates of calcium and magnesium makes water temporarily hard.
- The presence of sulphates and chlorides of magnesium and calcium makes water permanently hard.
Solution C. 5
- On heating, a saturated solution becomes unsaturated and more solute can be dissolved in the solution.
- By adding more solvent, a saturated solution can be made unsaturated.
Solution C. 6
Soap is chemically a sodium salt of stearic acid (an organic acid with the formula C17H35COOH) and has the formula C17H35COONa.
Soap is used for washing purposes.
Solution C. 7
Detergents are more soluble in water than soap and are unaffected by the hardness of water as their calcium salts are soluble in water.
Solution D. 1
Substances which contain water molecules along with salt are hydrated substances.
Examples: Sodium carbonate decahydrate: Na2CO3.10H2O
Copper sulphate pentahydrate: CuSO4.5H2O
Solution D. 2
The dissolved impurities in water are salts and minerals.
- Dissolved salts provide specific taste to water.
- Salts and minerals are essential for growth and development.
- They supply the essential minerals needed by our body.
Solution D. 3
- Advantages of soft water:
- When the water is soft, you use much less soap and fewer cleaning products. Your budget will reflect your savings.
- Plumbing will last longer. Soft water is low in mineral content and therefore does not leave deposits in the pipes.
- Clothes last longer and remain bright longer if they are washed in soft water.
- Advantages of hard water:
- Water free from dissolved salts has a very flat taste. The presence of salts in hard water makes it tasty. So, hard water is used in making beverages and wines.
- Calcium and magnesium salts present in small amounts in hard water are essential for bone and teeth development.
- Hard water checks the poisoning of water by lead pipes. When these pipes are used for carrying water, some lead salts dissolve in water to make it poisonous. Calcium sulphate present in hard water forms insoluble lead sulphate in the form of a layer inside the lead pipe and this checks lead poisoning.
Solution D. 4
In some limestone caves, conical pillar-like objects hang from the roof and some rise from the floor. These are formed by water containing dissolved calcium hydrogen carbonate continuously dropping from the cracks in the rocks. Release of pressure results in the conversion of some hydrogen carbonate to calcium carbonate.
Ca(HCO3)2 → CaCO3 + CO2 + H2O
This calcium carbonate little by little and slowly deposit on both roof and floor of the cave.
The conical pillar which grows downwards from the roof is called stalactite and the one which grows upward from the floor of the cave is called stalagmite.
These meet after a time. In a year, some grow less than even a centimetre, but some may be as tall as 100 cm.
CaCO3 + CO2 + H2O → Ca(HCO3)2
MgCO3 + CO2 + H2O → Mg(HCO3)2
If the water flows over beds of gypsum (CaSO4.2H2O), a little bit of gypsum gets dissolved in water and makes it hard.
Solution D. 5
- Ca(HCO3)2
 CaCO3 + H2O + CO2↑
CaCO3 + H2O + CO2↑
Mg(HCO3)2 ![]() MgCO3 + H2O + CO2↑
MgCO3 + H2O + CO2↑
- Ca(HCO3)2 + Ca(OH)2
 2CaCO3 + 2H2O
2CaCO3 + 2H2O
Mg(HCO3)2+ Ca(OH)2 ![]() MgCO3 + 2H2O
MgCO3 + 2H2O
Solution D. 6
- It is more difficult to form lather with soap.
- Scum may form in a reaction with soap, wasting the soap.
- Carbonates of calcium and magnesium form inside kettles. This wastes energy whenever you boil a kettle.
- Hot water pipes 'fur up'. Carbonates of calcium and magnesium start to coat the inside of pipes which can eventually get blocked.
Solution D. 7
- Steam is usually made in boilers which are made of a number of narrow copper tubes surrounded by fire. As the cold water enters these tubes, it is immediately changed into steam, while the dissolved solids incapable of changing into vapour deposit on the inner walls of the tubes. This goes on and makes the bore of the tubes narrower. The result is that less water flows through the tubes at one time and less steam is produced. When the bore of the tube becomes very narrow, the pressure of the steam increases so much that at times the boiler bursts.
- If hard water is used, calcium and magnesium ions of the water combine with the negative ions of the soap to form a slimy precipitate of insoluble calcium and magnesium usually called soap curd (scum).
Formation of soap curd will go on as long as calcium and magnesium ions are present. Till then, no soap lather will be formed and cleaning of clothes or body will not be possible. Moreover, these precipitates are difficult to wash from fabrics and sometimes form rusty spots if iron salts are present in water.
Solution D. 8
- Slaked lime
Ca(HCO3)2 + Ca(OH)2![]() 2CaCO3 + 2H2O
2CaCO3 + 2H2O
Mg(HCO3)2+ Ca(OH)2 ![]() MgCO3 + CaCO3 + 2H2O
MgCO3 + CaCO3 + 2H2O
Lime is first thoroughly mixed with water in a tank and then fed into another tank containing hard water. Revolving paddles thoroughly mix the two solutions. Most of the calcium carbonate settles down. If there is any solid left over, it is removed by a filter. This is known as Clarke's process.
- Washing soda
When washing soda or soda ash is added to hard water, the corresponding insoluble carbonates settle down and can be removed by filtration.
Ca(HCO3)2 + Na2CO3![]() CaCO3 + 2NaHCO3
CaCO3 + 2NaHCO3
Mg(HCO3)2+ Na2CO3![]() MgCO3 + 2NaHCO3
MgCO3 + 2NaHCO3
Solution D. 9
Permutit is an artificial zeolite. Chemically, it is hydrated sodium aluminium orthosilicate with the formula Na2Al2Si2O8.XH2O. For the sake of convenience, let us give it the formula Na2P.
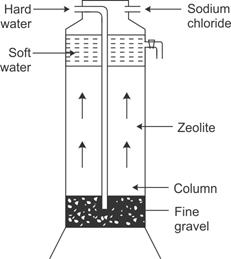
A tall cylinder is loosely filled with lumps of permutit. When hard water containing calcium and magnesium ions percolates through these lumps, ions exchange. Sodium permutit is slowly changed into calcium and magnesium permutit, and the water becomes soft with the removal of calcium and magnesium ions.
When no longer active, permutit is regenerated by running a concentrated solution of brine over it and removing calcium chloride formed by repeated washing.
CaP + 2NaCl → Na2P + CaI2
Solution E. 1
(a) Identify and label the curves with the salt it represents.
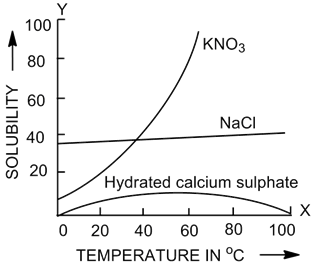
(b) The factors on which the solubility depends are as follows:
(i) Size of solute particles: The smaller the size of the solute particles, the greater is its total surface area exposed to the solvent. Therefore, the greater is the solubility of that solute.
Hydrated calcium sulphate
(ii) Stirring: This brings more of the solvent in contact with the solute and thus increases the rate of formation of solution.
(iii) Temperature: The solubility of a gas in a liquid always decreases with rise in temperature. But the solubility of most solids in water usually increases with rise in temperature.
For example, the solubility of potassium nitrate in water at 20°C is 31.6 grams, whereas its solubility at 60°C is 108 grams.
All these factors affect solubility because in the process of dissolution, the particles of solute merely occupy the spaces between the particles of solvent without undergoing any chemical change.
(c) Solubility of which salt(s) shows:
(i) Solubility of KNO3 salt shows endothermic process.
(ii) Solubility of hydrated calcium sulphate salt shows exothermic process.