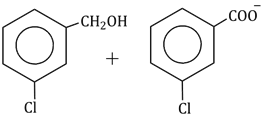
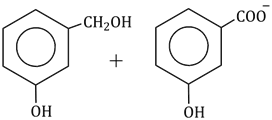
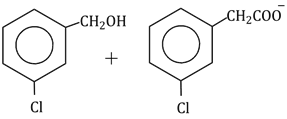
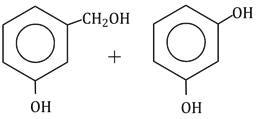
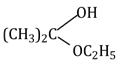Question Paper (Section wise)
-
1) If force (F), velocity (V) and time (T) are taken as fundamental units, then the dimensions of mass are:-
-
[F V T-1]
-
[F V T-2]
-
[F V-1 T-1]
-
[F V-1 T]
-
-
2) A projectile is fired from the surface of the earth with a velocity of 5 ms-1 and angle θ with the horizontal. Another projectile fired from another planet with a velocity of 3 ms-1 at the same angle follows a trajectory while is identical with the trajectory of the projectile fired from the earth. The value of the acceleration due to gravity on the planet is (in ms-2) is: (given g = 9.8 m/s2)
-
3.5
-
5.9
-
16.8
-
110.8
-
-
3) A particle is moving such that its position coordinate (x, y) are
(2m, 3m) at time t = 0
(6m, 7m) at time t = 2 s and
(13m, 14) at time t = 5 s
Average velocity
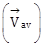 from t = 0 to t = 5 s is
from t = 0 to t = 5 s is -
-
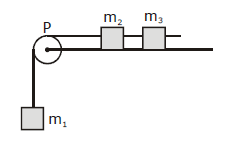
4) A system consists of three masses m1, m2 and m3 connected by a string passing over a pulley P. The mass m1 hangs freely and m2 and m3 are on a rough horizontal table (the coefficient of friction = μ). The pulley is frictionless and of negligible mass. The downward acceleration of mass m1 is (Assume m1 = m2 = m3 = m)
-
-
5) The force ‘F acting on a particle of mass ‘m’ is indicated by the force – time graph shown below. The change in momentum of the particle over the time Interval from zero to 8 s Is:
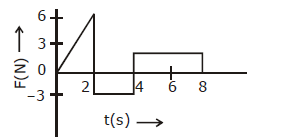
-
24 N s
-
20 N s
-
12 N s
-
6 N s
-
-
6) A balloon with mass ‘m’ is descending down with an acceleration ‘a’ (where a < g). How much mass should be removed from it so that it starts moving up with an acceleration ‘a’?
-
-
7) A body of mass (4m) is lying in x-y plane at rest. It suddenly explodes into three pieces. Two pieces, each of mass (m) move perpendicular to each other with equal speeds (v), The total kinetic energy generated due to explosion is
-
mv2
-
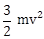
-
2mv2
-
4mv2
-
-
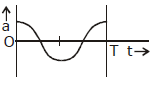
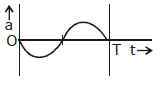
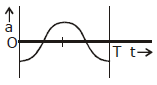
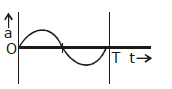
8) The oscillation of body on a smooth horizontal surface is represented by the equation,
X = A cos (ωt)
Where
X = displacement at time t
ω = frequency of oscillation
Which one of the following graphs shows correctly the variation ‘a’ with ‘t’?
-
-
9) A solid cylinder of mass 50 kg and radius 0.5 m is free to rotate about the horizontal axis. Massless string is wound round the cylinder with one end attached to it and other hanging freely. Tension in the string required to produce an angular acceleration of 2 revolutions S-2 is :-
-
25 N
-
50 N
-
78.5 N
-
157 N
-
-
10) In Young’s double slit experiment, the slits are 2mm apart and are illuminated by photons of two wavelength λ1 = 12000 Å and λ2 = 10000 Å. At what minimum distance from the common central bright fringe on the screen 2m from the slit will a bright fringe from one interference pattern coincide with a bright fringe from the other?
-
3 mm
-
8 mm
-
6 mm
-
4 mm
-
-
11) In a common emitter (CE) amplifier having a voltage gain G, transistor used has trans conductance 0.02 mho and current gain 20, the voltage gain will be-
-
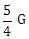
-
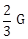
-
1.5 G
-
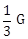
-
-
12) A certain mass of hydrogen is changed to Helium by the process of fusion. The mass defect in fusion reaction is 0.02866 u. The energy liberated per u is : (given 1u = 931 MeV)
-
13.35 MeV
-
2.67 MeV
-
26.7 MeV
-
6.675 MeV
-
-
13) In the given (V-T) diagram, what is the relation between pressure P1 and P2?
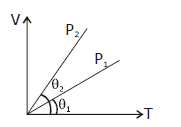
-
Cannot be predicted
-
P2 = P1
-
P2 > P1
-
P2 < P1
-
-
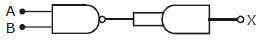
14) The output (X) of the logic circuit shown in figure will be:
-
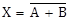
-
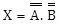
-
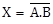
-
X = A.B
-
-
15) Three blocks with masses m, 2m and 3m are connected by strings, as shown in the figure. After an upward force F is applied on block m, the masses move upward at constant speed v. What is the net force on the block of mass 2m? (g is the acceleration due to gravity)
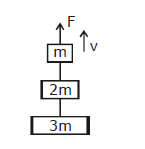
-
6 mg
-
zero
-
2 mg
-
3 mg
-
-
16) In a n-type semiconductor, which of the following statement is true:
-
Holes are majority carriers and trivalent atoms are dopants
-
Electrons are majority carriers and trivalent atoms are dopants
-
Electron are minority carriers and pentavalent atoms are dopants
-
Holes are minority carriers and pentavalent atoms are depends.
-
-
17) The half life of radioactive isotope ‘X’ is 20 years. It decays to another element ‘Y’ which is stable. The two elements ‘X’ and ‘Y’ were found to be in the ratio 1:7 in a sample of a given rock. The age of the rock is estimated to be:
-
100 years
-
40 years
-
60 years
-
80 years
-
-
18) The molar specific heats of an ideal gas at constant pressure and volume are denoted by Cp and Cv’ respectively. If
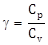 and R is the universal gas constant, then Cv is equal to:
and R is the universal gas constant, then Cv is equal to: -
-
19) The damping force on an oscillator is directly proportional to the velocity. The unit of the constant of proportionality are:-
-
kgs-1
-
kgs
-
kgms-1
-
kgms-2
-
-
20) The motion of a particle along a straight line is described by equation x = 8 + 12t – t3 where x is in metre and t in second. The retardation of the particle when its velocity becomes zero is:-
-
6 ms-2
-
12 ms-2
-
24 ms-2
-
zero
-
-
21) The horizontal range and the maximum height of a projectile are equal. The angle of projection of the projectile is:-
-
θ = tan-1 (2)
-
θ = 45°
-
θ = tan-1
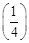
-
θ = tan -1 (4)
-
-
22) A particle has initial velocity
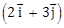 and acceleration
and acceleration 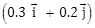 . The magnitude of velocity after 10 second will be:
. The magnitude of velocity after 10 second will be:-
5 units
-
9 units
-
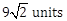
-
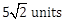
-
-
23) A car of mass 1000 kg negotiates a banked curve of radius 90m on a frictionless road. If the banking angle is 45°, the speed of the car is:
-
5ms-1
-
10ms-1
-
20ms-1
-
30ms-1
-
-
24) A solid cylinder of mass 3 kg is rolling on a horizontal surface with velocity 4 ms-1. It collides with a horizontal spring of force constant 200 Nm-I. The maximum compression produced in the spring will be:
-
0.7 m
-
0.2 m
-
0.5 m
-
0.6 m
-
-
25) The potential energy of a particle in a force field is:
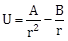 where A and B are positive constants and r is the distance of particle from the centre of the field. For stable equilibrium, the distance of the particles is:
where A and B are positive constants and r is the distance of particle from the centre of the field. For stable equilibrium, the distance of the particles is:-
A/B
-
B/A
-
B/2A
-
2A/B
-
-
26) Two spheres A and B of masses m1 and m2 respectively collide. A is at rest initially and B is moving with velocity v along x-axis. After collision B has a velocity v/2 in a direction perpendicular to the original direction. The mass A moves after collision in the direction.
-
θ = tan-1 (1/2) to the x-axis
-
θ = tan-1 (-1/2) to the x-axis
-
same as that of B
-
opposite to that of B
-
-
27) Two persons of masses 55 kg and 665 kg respectively, are at the opposite ends of a boat. The length of the boat is 3.0 m and weight 100 kg. The 55 kg man walks up to the 65 kg man and sits with him. If the boat is in still water the centre of mass of the system shifts by:
-
zero
-
0.75 m
-
3.0 m
-
2.3 m
-
-
28) The fundamental frequency in an open organ pipe is equal to the third harmonic of a closed organ pipe if the length of the closed organ pipe is 20 cm, the length of the open organ pipe is
-
12.5 cm
-
8 cm
-
13.2 cm
-
16 cm
-
-
29) The figure shown a logic circuit two inputs A and B and the output C. The voltage wave forms across circuit gate is:
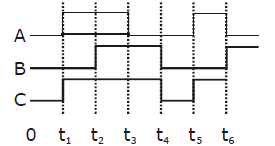
-
AND gate
-
NAND gate
-
OR gate
-
NOR gate
-
-
30) In a CE transistor amplifier, the audio signal voltage across the collector resistance of 2kΩ is 2V. If the base resistance is 1kΩ and the current amplification of the transistor is 100, the input signal voltage is:-
-
1 mV
-
10mV
-
0.1 V
-
1.0 V
-
-
31) C and Si both have same lattice structure, having 4 bonding electrons in each. However, C is insulator where as Si is intrinsic semiconductor. This is because:
-
The four bonding electron in the case of C lie in the second orbit, whereas in the case of Si they lie in the third
-
The four bonding electron in the case of C lie in the third orbit, whereas for Si they lie in the fourth orbit.
-
In case of C the valance band is not completely filled at absolute zero temperature.
-
In case of C the conduction band is partly filled even at absolute zero temperature
-
-
32) Transfer characteristics (output voltage (V0) vs Input voltage (Vi) for a base biased transistor in CE configuration is as shown in the figure. For using transistor as a switch, it is used.
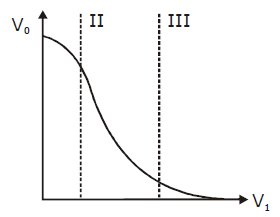
-
in region II
-
in region I
-
in region III
-
both in region (I) and (III)
-
-
33) A magnetic needle suspended parallel to a magnetic field requires
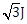 of work to turn it through 60°. The torque needed to maintain the needle in this position will be:
of work to turn it through 60°. The torque needed to maintain the needle in this position will be:-
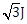
-
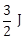
-
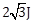
-
3J
-
-
34) The transition from the state n = 3 to n = 1 in a hydrogen like atom results in ultraviolet radiation. Infrared radiation will be obtained in the transition from:
-
5 → 4
-
4 → 3
-
2 → 1
-
3 → 2
-
-
35) The power dissipated in the circuit shown in the figure is 30 watts. The value of R is-
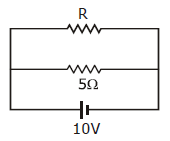
-
10 Ω
-
30 Ω
-
20 Ω
-
15 Ω
-
-
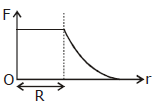
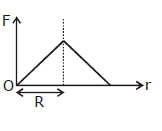
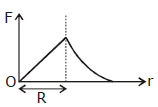
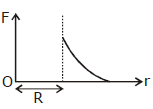
36) Which one of the following plots represents the variation of gravitational field on a particle with distance due to a thin spherical shell of radius R? (r is measured from the center of the spherical shell)
-
-
37) The potential energy of a system increases if work is doneyu
-
Upon the system by a conservative force
-
Upon the system by a non- conservative force
-
By the system against a conservative force
-
By the system against a non-conservative force
-
-
38) A body is moving with velocity 30 m/s towards east. After 10 seconds its velocity becomes 40 m/s towards north. The average acceleration of the body is-
-
5 m/s2
-
1 m/s2
-
7 m/s2
-
7 m/s2
-
-
39) A missile is fired for maximum range with an initial velocity of 20 m/s. If g = 10 m/s2, the range of the missile is-
-
20 m
-
40 m
-
50 m
-
60 m
-
-
40) Force F on a particle moving in a straight line varies with distance d as shown in the figure. The work done on the particle during its displacement of 12 m is-
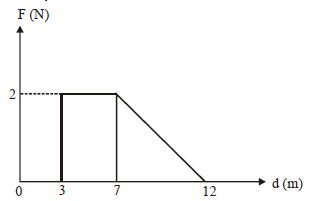
-
13 J
-
18 J
-
21 J
-
26 J
-
-
41) A charge Q is enclosed by a Gaussian spherical surface of radius R. If the radius is doubled. then the outward electric flux will
-
Be doubled
-
Increases four times
-
Be reduced to half
-
Remain the same
-
-
42) Four electric charges +q, +q, -q and –q are placed at the corners of a square of side 2L (see figure). The electric potential at point A, midway between the two charges +q and +q, is-
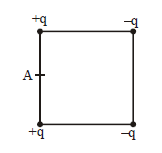
-
-
43) A parallel plate condenser has a uniform electric field E (V/m) in the space between the plates. If the distance between the plates is d (m) and area of each plate is A (m2) the energy (joules) stored in the condenser is-
-
-
44) If power dissipated in the 9Ω resistor in the circuit shown is 36 Watt, the potential difference across the 2Ω resistor is-
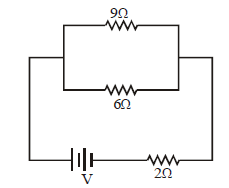
-
2 Volt
-
4 Volt
-
8 Volt
-
10 Volt
-
-
45) A current of 2A flows through a 2Ω resistor when connected across a battery. The same battery supplies a current of 0.5 A when connected across a 9Ω resistor. The internal resistance of the battery is
-
1 Ω
-
0.5 Ω
-
1/3 Ω
-
¼ Ω
-
-
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
-
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
-
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
-
1) What is the maximum number of orbitals that can be identified with the following quantum numbers?
n = 3, 𝓁 = 1, m𝓁 = 0
-
1
-
2
-
3
-
4
-
-
2) Calculate the energy in joule corresponding to light of wavelength 45 nm: (planck's constant h = 6.63 × 10–34 Js; speed of light c = 3 × 108 ms–1)
-
6.67 × 1015
-
6.67 × 1011
-
4 .42 × 10–15
-
4.42 × 10–18
-
-
3) Equal masses of H2, O2 and methane have been taken in a container of volume V at temperature 27°C in identical conditions. The ratio of the volumes of gases H2: O2: methane would be:
-
8 : 16 : 1
-
16 : 8 : 1
-
16 : 1 : 2
-
8 : 1 : 2
-
-
4) If a is the length of the side of a cube, the distance between the body centered atom and one corner atom in the cube will be:
-
-
5) Which property of colloids is not dependent on the charge on colloidal particles?
-
Coagulation
-
Electrophoresis
-
Electro–osmosis
-
Tyndall effect
-
-
6) Which of the following salts will give highest pH in water?
-
KCl
-
NaCl
-
Na2CO3
-
CuSO4
-
-
7) Which of the following 0.10m aquous solutions, which one will exhibit the largest freezing point depression?
-
KCl
-
C6H12O6
-
Al2(SO4)3
-
K2SO4
-
-
8) When 22.4 litres of H2 (g) is mixed with 11.2 litres of Cl2 (g), each at S.T. P., the moles of HCl (g) formed is equal to:
-
1 mol of HCl (g)
-
2 mol of HCl (g)
-
0.5 mol of HCl
-
1.5 mol of HCl (g)
-
-
9) When 0.1 mol MnO42– is oxidised the quantity of electricity required to completely oxidise MnO42– to MnO4– is: –
-
96500 C
-
2 × 96500 C
-
9650 C
-
96.50 C
-
-
10) The value of Planck’s constant is 6.63 × 10-34 Js. The speed of light is 3 × 1017 nm s-1. Which value is closets to the wavelength in nanometer of a quantum of light with frequency of 6 × 1015 s-1?
-
75
-
10
-
25
-
50
-
-
11) The radical,
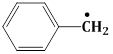 is a aromatic
is a aromaticBecause it has:
-
6p-orbital and 7 unpaired electrons
-
6p-orbital and 6 unpaired electrons
-
7p-orbital and 6 unpaired electrons
-
7p-orbital and 7 unpaired electrons
-
-
12) Which of the following is electron-deficient?
-
PH3
-
(CH3)2
-
(SiH3)2
-
(BH3)2
-
-
13) Which of the following statements about the interstitial compounds is incorrect?
-
They have higher melting points than the pure metal
-
They retain metallic conductive
-
They are chemically reactive
-
They are much harder than the pure metal
-
-
14) How many grams of concentrated nitric acid solution should be used to prepare 250 mL of 2.0 M HNO3?
-
54. 0 conc. HNO3
-
45.0 conc. HNO3
-
90.0 conc. HNO3
-
70.0 conc. HNO3
-
-
15) Which of the following lanthanoid ions is diamagnetic?
(At. No., Ce = 58, Sm = 62, Vb = 70)
-
Yb2+
-
Ce2+
-
Sm2+
-
Eu2+
-
-
16) Which one of the following molecules contains no π bond?
-
NO2
-
CO2
-
H2O
-
SO2
-
-
17) Based on equation E = − 2.178 × 10-18 certain conclusions are written. Which of them is not correct?
-
For n = 1, the electron has a more negative energy than it does for n = 6 which means that the electron is more loosely bound in the smallest allowed orbit.
-
The negative sign in equation simply means that the energy of electron bound to the nucleus is lower than it would be if the electrons were at the infinite distance from the nucleus
-
Larger the value of n, the larger is the orbit radius
-
Equation can be used to calculate the change in energy when the electron change orbit
-
-
18) In the reaction
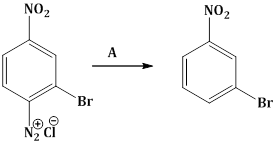
A is
-
H+/H2O
-
HgSO4/H2SO4
-
Cu2Cl2
-
H3PO2 and H2O
-
-
19) In a zero-order reaction for every 10° rise of temperature, the rate is doubled. If the temperature is increased from 10°C to 100°C, the rate of the reaction will become:
-
64 times
-
128 times
-
256 times
-
512 times
-
-
20) Which one of the following pairs is isostructural (i.e. having the same shape and hybridization)?
-
[NF3 and BF3]
-
[
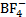 and
and 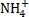 ]
] -
[BCl3 and BrCl3]
-
[NH3 and
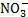 ]
]
-
-
21) In which of the following reactions, standard reaction entropy charge (∆S°) is positive and standard Gibb’s energy charge (∆G°) decreases sharply with increasing temperature?
-
-
22) In a reaction A + B → Product, rate is doubled when the concentration of B is doubled and rate increased by a factor of 8 when the concentration of both the reactants (A and B) are doubled, rate law for the reaction can be written as:
-
Rate = k [A] [B]
-
Rate = [A]2 [B]
-
Rate = k [A] [B]2
-
Rate = k [A]2 [B]2
-
-
23) Limiting molar conductivity of NH4OH
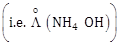 is equal to :
is equal to : -
-
24) Which of the following species contains three bond pairs and one lone pair around the central atom?
-
NH4–
-
PCl3
-
H2O
-
BF3
-
-
25) Buffer solutions have constant acidity and alkalinity because:
-
they have large excess of H+ or OH- ions
-
they have fixed value of pH
-
these give unionized acid or base on reaction with added acid or alkali
-
acids and alkalies in these solutions are sheded from attack by other ions
-
-
26) In Freundlich Adsorption isotherm, the value of 1/n is:
-
1 in case of physical adsorption
-
1 in case of chemisoption
-
between 0 and 1 in all cases
-
between 2 and 4 in all cases
-
-
27) pH of a saturated solution of Ba (OH2) is 12. The value of solubility product (Ksp) of Ba (OH)2 is:
-
4.0 × 10-6
-
5.0 × 10-6
-
3.3 × 10-7
-
5.0 × 10-7
-
-
28) Predict the products in the given reaction:
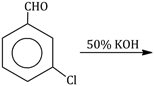
-
-
29) Which of the following acids does not exhibit optical isomerism?
-
Lactic acid
-
Tertaric acid
-
Maleic acid
-
α- amino acids
-
-
30) CH3 CHO and C6H5CHO can be distinguished chemically by:
-
Tollen’s reagent test
-
Fehling solution test
-
Benedict test
-
Iodoform test
-
-
31) Which of the following statements is false?
-
The repeat unit in natural rubber is isoprene
-
Both starch and cellulose are polymers of glucose
-
Artificial silk is derived from cellulose
-
Nylon-66 is an example of elastomer
-
-
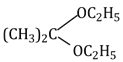
32) Acetone is treated with excess of ethanol is the presence of hydrochloric acid. The product obtained is:
-
-
33) The Gibbs’ energy for the decomposition of Al2O3 at 500° C is as follows:
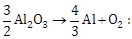
∆r G = + 960 kJ mol-1
The potential difference needed for the electrolytic reduction of aluminium oxide
(Al2O3) at 500°C is at least:
-
2.5 V
-
5.0 V
-
4.5 V
-
3.0 V
-
-
34) Which of the following compounds can be used as antifreeze in automobile radiators?
-
Nitrophenol
-
Ethyl alcohol
-
Methyl alcohol
-
Glycol
-
-
35) A certain gas takes three times as long to effuse out as helium. Its molecular mass will be:
-
64 u
-
9 u
-
27 u
-
36 u
-
-
36) Vapour pressure of chloroform (CHCl3) and dichloromethane (CH2Cl2) at 25°C are 200 mmHg and 415 mmHg respectively. Vapour pressure of the solution obtained by mixing 25.5 g of CHCl3 and 40g of CH2Cl2 at the same temperature will be:
(Molecular mass of CH2Cl2 = 85 u)
-
347.9 mmHg
-
285.5 mmHg
-
173.9 mmHg
-
615 mmHg
-
-
37) The van’t Hoff factor, if a compound which undergoes dissociation in one solvent and association in other solvent is respectively-
-
Greater than one and greater than one
-
Less than one and greater than one
-
Less than one and less than one
-
Greater than one and less than one
-
-
38) Standard electrode potential for Sn4+/Sn2+ couple is +0.15V and that for the Cr3+/Cr couple is -0.74 V. These two couples in their standard state are connected to make a cell. The potential will be
-
+1.83 V
-
+1.19 V
-
+0.89 V
-
+0.18 V
-
-
39) A gaseous mixture was prepared by taking equal mole of CO and N2. If the total pressure of the mixture was found 1 atmosphere, the partial pressure of the nitrogen (N2) in the mixture is
-
1 atm
-
0.5 atm
-
0.8 atm
-
0.9 atm
-
-
40) If the E0cell for a given reaction has a negative value, then which of the following gives the correct relationship for the values of ∆G° and Keq?
-
∆G° > 0; Keq < 1
-
∆G° > 0; Keq > 1
-
∆G° < 0; Keq > 1
-
∆G° < 0; Keq < 1
-
-
41) The freezing point depression constant for water is -1.86 °Cm-1. If 5.00g Na2SO4 is dissolved in 45.0 g H2O, the freezing point is changed by -3.82°C. Calculate the van’t Hoff factor Na2SO4.
-
0.381
-
2.05
-
2.63
-
3.11
-
-
42) The energies E1 and E2 of two radiation are 25 eV and 50 eV respectively. The relation between their wavelengths i.e. λ1 and λ2 will be-
-
-
43) Standard electrode potential o three metals X, Y, and Z are -1.2 V, + 0.5 V and -3.0 V Respectively. The reducing power of these metals will be
-
X > Y > Z
-
Y > Z > X
-
Y > X > Z
-
Z > X > Y
-
-
44) Which one of the following statements for the order of a reaction is incorrect?
-
Order of reaction is always whole number
-
Order can be determined only experimentally
-
Order is not influenced by stoichiometric coefficient of the reactants
-
Order of reaction is sum of power to the concentration terms of reactants to express the rate of reaction
-
-
45) Enthalpy change for the reaction, 4H (g) → 2H2(g) is
-869.6 kJ. The dissociation energy of H –H bond is-
-
+217.4 kJ
-
-434.8 kJ
-
-869.6 kJ
-
+434.8 kJ
-
-
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
-
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
-
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
-
1) Five kingdom system of classification suggested by R.H. Whittaker is not based on:
-
Mode of reproduction
-
Mode of nutrition
-
Complexity of body organisation
-
Habitat
-
-
2) The main function of mammalian corpus luteum is to produce:
-
Progesterone
-
Human chorionic gonadotropin
-
Relaxin only
-
Estrogen only
-
-
3) In which one of the following processes CO2 is not released?
-
Aerobic respiration in animals
-
Alcoholic fermentation
-
Lactate fermentation
-
Aerobic respiration in plants
-
-
4) Choose the correctly matched pair:
-
Moist surface of buccal cavity – Glandular epithelium
-
Tubular parts of nephrons – Cuboidal epithelium
-
Inner surface of bronchioles – Squamous epithelium
-
Inner lining of salivary ducts – Ciliated epithelium
-
-
5) Which of the following shows coiled RNA strand and capsomeres?
-
Tobacco mosaic virus
-
Measles virus
-
Retrovirus
-
Polio virus
-
-
6) Just as a person moving from Delhi to Shimla to escape the heat for the duration of hot summer, thousands of migratory birds from Siberia and other extremely cold northern regions move to:
-
Meghalaya
-
Corbett National Park
-
Keoladeo National Park
-
Western Ghat
-
-
7) You are given a fairly old piece of dicot stem and a dicot root. Which of the following anatomical structures will you use to distinguish between the two?
-
Secondary phloem
-
Protoxylem
-
Cortical cells
-
Secondary xylem
-
-
8) In ‘S’ phase of the cell cycle:
-
The amount of DNA remains same in each cell.
-
The chromosome number is increased.
-
The amount of DNA is reduced to half in each cell.
-
The amount of DNA doubles in each cell.
-
-
9) A species facing extremely high risk of extinction in the immediate future is called:
-
Endemic
-
Critically endangered
-
Extinct
-
Vulnerable
-
-
10) Fruit colour in squash is an example of:
-
Dominant epistasis
-
Complementary genes
-
Inhibitory genes
-
Recessive epistasis
-
-
11) Identify the hormone with its correct matching of source and function.
-
Melatonin – Pineal gland – regulates the normal rhythm of sleep-wake cycle.
-
Progesterone – corpus luteum – secondary sex organs.
-
Atrial natriuretic factor –ventricular wall – increases the blood pressure.
-
Oxytocin – posterior pituitary – growth and maintenance of mammary glands.
-
-
12) An example of edible underground stem is
-
Groundnut
-
Sweet potato
-
Potato
-
Carrot
-
-
13) Which of the following causes an increase in sodium reabsorption in the distal convoluted tubule?
-
Increase in antidiuretic hormone levels
-
Decrease in aldosterone levels
-
Decrease in antidiuretic hormone levels
-
Increase in aldosterone levels
-
-
14) Which structures perform the function of mitochondria in bacteria?
-
Ribosomes
-
Cell wall
-
Mesosomes
-
Nucleoid
-
-
15) Select the option which is not correct with respect to enzyme action:
-
Addition of lot of succinate does not reverse the inhibition of succinic dehydrogenase by malonate.
-
A non-competitive inhibitor binds the enzyme at a site distinct from that which binds the substrate.
-
Malonate is a competitive inhibitor of succinic dehydrogenase.
-
Substrate binds with the enzyme at its active site.
-
-
16) Which is the particular type of drug that is obtained from the plant whose one flowering branch is shown below?

-
Depressant
-
Stimulant
-
Pain-killer
-
Hallucinogen
-
-
17) Fructose is absorbed into the blood through mucosa cells of intestine by the process called:
-
Facilitated transport
-
Simple diffusion
-
Co-transport mechanism
-
Active transport
-
-
18) The solid linear cytoskeletal elements having a diameter of 6 nm and made up of a single type of monomer are known as
-
Microfilaments
-
Intermediate filaments
-
Lamins
-
Microtubules
-
-
19) Person with blood group AB is considered as universal recipient because he has:
-
Both A and B antibodies in the plasma.
-
No antigen on the RBC and no antibody in the plasma.
-
Both A and B antigens in the plasma but no antibodies.
-
Both A and B antigens on the RBC but no antibodies in the plasma.
-
-
20) Select the wrong statement:
-
Isogametes are similar in structure, function and behaviour.
-
Anisogametes differ either in structure, function or behaviour.
-
In oomycetes female gamete is smaller and motile, while male gamete is larger and non-motile.
-
Chlamydomonas exhibits both isogamy and anisogamy and Fucus shows oogamy.
-
-
21) Which one of the following is not a correct statement?
-
Herbarium houses dried, pressed and preserved plant specimens.
-
Botanical gardens have a collection of living plants for reference.
-
A museum has a collection of photographs of plants and animals.
-
Key is a taxonomic aid for the identification of specimens.
-
-
22) Isogamous condition with non-flagellated gametes is found in
-
Chlamydomonas
-
Spirogyra
-
Volvox
-
Fucus
-
-
23) Besides paddy fields, cyanobacteria are also found inside vegetative part of
-
Pinus
-
Cycas
-
Equisetum
-
Psilotum
-
-
24) Megasporangium is equivalent to
-
Embryo sac
-
Fruit
-
Nucellus
-
Ovule
-
-
25) Read the following statements (A-E) and answer the question which follows them.
- In liverworts, mosses, and ferns gametophytes are free-living.
- Gymnosperms and some ferns are heterosporous.
- Sexual reproduction in Fucus, Volvox and Albugo is oogamous.
- The sporophyte in liverworts is more elaborate than that in mosses.
- Both Pinus and Marchantia are dioecious.
How many of the above statements are correct?
-
One
-
Two
-
Three
-
Four
-
26) Among bitter gourd, mustard, brinjal, pumpkin, china rose, lupin, cucumber, sunn hemp, gram, guava, bean, chilli, plum, Petunia, tomato, rose, Withania, potato, onion, aloe, and tulip how many plants have hypogynous flower?
-
Six
-
Ten
-
Fifteen
-
Eighteen
-
-
27) Interfascicular cambium develops from the cells of
-
Medullary rays
-
Xylem parenchyma
-
Endodermis
-
Pericycle
-
-
28) In China rose the flowers are
-
Actinomorphic, hypogynous with twisted aestivation
-
Actinomorphic, epigynous with valvate aestivation
-
Zygomorphic, hypogynous with imbricate aestivation
-
Zygomorphic, epigynous with twisted aestivation
-
-
29) Lenticels are involved in
-
Transpiration
-
Gaseous exchange
-
Food transport
-
Photosynthesis
-
-
30) Age of a tree can be estimated by
-
Its height and girth
-
Biomass
-
Number of annual rings
-
Diameter of its heartwood
-
-
31) Seed coat is not thin, membranous in
-
Maize
-
Coconut
-
Groundnut
-
Gram
-
-
32) Transition state structure of the substrate formed during an enzymatic reaction is
-
Transient but stable
-
Permanent but unstable
-
Transient but unstable
-
Permanent and stable
-
-
33) A phosphoglyceride is always made up of
-
Only a saturated fatty acid esterified to a glycerol molecule to which a phosphate group is also attached.
-
Only an unsaturated fatty acid esterified to a glycerol molecule to which a phosphate group is also attached.
-
A saturated or unsaturated fatty acid esterified to a glycerol molecule to which a phosphate group is also attached.
-
A saturated or unsaturated fatty acid esterified to a phosphate group which is also attached to a glycerol molecule.
-
-
34) Pigment-containing membranous extensions in some cyanobacteria are
-
Heterocysts
-
Basal bodies
-
Pneumatophores
-
Chromatophores
-
-
35) A major site for synthesis of lipids is
-
RER
-
SER
-
Symplast
-
Nucleoplasm
-
-
36) The complex formed by a pair of synapsed homologous chromosomes is called
-
Equatorial plate
-
Kinetochore
-
Bivalent
-
Axoneme
-
-
37) Global warming can be controlled by
-
Reducing deforestation, cutting down the use of fossil fuel.
-
Reducing reforestation, increasing the use of fossil fuel.
-
Increasing deforestation, slowing down the growth of human population.
-
Increasing deforestation, reducing efficiency of energy usage.
-
-
38) The Air Prevention and Control of Pollution Act came into force in
-
1975
-
1981
-
1985
-
1990
-
-
39) Cycas and Adiantum resemble each other in having:
-
Cambium
-
Vessels
-
Seeds
-
Motile sperms
-
-
40) Gymnosperms are also called soft wood spermatophytes because they lack:
-
Thick-walled tracheids
-
Xylem fibres
-
Cambium
-
Phloem fibres
-
-
41) Maximum nutritional diversity is found in the group:
-
Monera
-
Plantae
-
Fungi
-
Animalia
-
-
42) Which one of the following is common to multicellular fungi, filamentous algae and protonema of mosses?
-
Mode of nutrition
-
Multiplication by fragmentation
-
Diplontic life cycle
-
Members of Kingdom Plantae
-
-
43) Which statements is wrong for viruses?
-
They have the ability to synthesise nucleic acids and proteins.
-
Antibiotics have no effect on them.
-
All are parasites.
-
All of them have helical symmetry.
-
-
44) Which one of the following is a correct statement?
-
Antheridiophores and archegoniophores are present in pteridophytes.
-
Origin of seed habit can be traced in pteridophytes.
-
Pteridophyte gametophyte has a peritoneal and leafy stage.
-
In gymnosperms female gametophyte is free living.
-
-
45) Nuclear membrane is absent in:
-
Volvox
-
Penicillium
-
Nostoc
-
Agaricus
-
-
46) During gamete formation, the enzyme recombinase participates during:
-
Prophase-I
-
Prophase-II
-
Metaphase-I
-
Anaphase-II
-
-
47) Which one of the following does not differ in E.coli and Chlamydomonas?
-
Cell wall
-
Cell membrane
-
Ribosomes
-
Chromosomal organisation
-
-
48) PCR and Restriction Fragment Length Polymorphism are the methods for:-
-
DNA sequencing
-
Genetic fingerprinting
-
Study of enzymes
-
Genetic transformation
-
-
49) Removal of RNA polymerase III from nucleoplasm will affect the synthesis of:
-
m-RNA
-
r-RNA
-
t-RNA
-
hn-RNA
-
-
50) Evolution of different species in a given area starting from a point and spreading to other geographical area is known as:
-
Migration
-
Divergent evolution
-
Adaptive radiation
-
Natural selection
-
-
51) Removal of introns and joining of exons in a defined order during transcription is called:
-
Slicing
-
Splicing
-
Looping
-
Inducing
-
-
52) Which one of the following is not a part of the transcription unit in DNA?
-
A promoter
-
The structural gene
-
The inducer
-
A terminator
-
-
53) An organic substance that can withstand environmental extreme and cannot be degraded by any enzymes is:
-
Lignin
-
Cellulose
-
Cuticle
-
Sporopollenin
-
-
54) Best defined function of manganese in green plants is:
-
Nitrogen fixation
-
Water absorption
-
Photolysis of water
-
Calvin cycle
-
-
55) Common cold differs from pneumonia in that:
-
Pneumonia in caused by a virus while common cold is caused by the bacterium Haemophilus influenzae.
-
Pneumonia pathogen infects alveoli whereas common cold affects nose and respiratory passage but not the lungs.
-
Pneumonia is a communicable disease whereas common cold is a nutritional deficiency disease.
-
Pneumonia can be prevented by a live attenuated bacterial vaccine whereas common cold has no effective vaccine.
-
-
56) Identify the possible link “A” in the following food chain:
Plant → Insect → Frog → “A” → Eagle
-
Cobra
-
Parrot
-
Rabbit
-
Wolf
-
-
57) Which one of the following is an example of carrying out biological control of pests/diseases using microbes?
-
Bt-cotton to increases cotton yield.
-
Lady bird beetle against aphids in mustard.
-
Trichoderma sp. against certain plant pathogens.
-
Nucleopolyhedrovirus against white rust in Brassica.
-
-
58) Widal Test is carried out to test:
-
HIV/AIDS
-
Typhoid fever
-
Malaria
-
Diabetes mellitus
-
-
59) Cirrhosis of liver is caused by the chronic intake of:
-
Tobacco (Chewing)
-
Cocaine
-
Opium
-
Alcohol
-
-
60) Which one of the following in not a property of cancerous cells whereas the remaining three are?
-
They divide in an uncontrolled manner.
-
They show contact inhibition.
-
They compete with normal cells for vital nutrients.
-
They do not remain confined in the area of formation.
-
-
61) Motile zygote of Plasmodium occurs in:
-
Human RBCs
-
Human liver
-
Gut of female Anopheles
-
Salivary glands of Anopheles
-
-
62) In which one of the following options the two examples are correctly matched with their particular type of immunity?
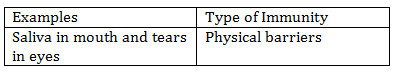
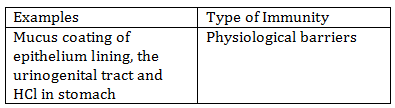
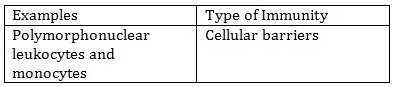
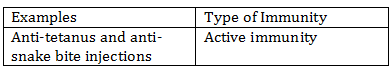
-
-
63) The figure below is the diagrammatic representation of the E.coli vector pBR 322. Which one of the given options correctly identifies its certain component(s)?
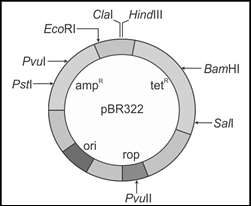
-
Hind III, EcoRI - selectable markers
-
ampR, tetR - antibiotic resistance genes
-
ori - original restriction enzyme
-
rop - reduced osmotic pressure
-
-
64) Measuring Biochemical Oxygen Demand (BOD) is a method used for:
-
Measuring the activity of Saccharomyces cerevisiae in producing curd on a commercial scale.
-
Working out the efficiency of RBCs about their capacity to carry oxygen.
-
Estimating the amount of organic matter in sewage water.
-
Working out the efficiency of oil driven automobile engines.
-
-
65) The most abundant prokaryotes helpful to humans in making curd from milk and in production of antibiotics are the ones categorised as:
-
Chemosynthetic autotrophs
-
Heterotrophic bacteria
-
Cyanobacteria
-
Archaebacteria
-
-
66) People who have migrated from planes to an area adjoining Rohtang pass about six months back:
-
Suffer from altitude sickness with symptoms like nausea, fatigue, etc.
-
Have the usual RBC count but their haemoglobin has very high binding affinity to O2
-
Have more RBCs and their haemoglobin has a lower binding affinity to O2.
-
Are not physically fit to play games like football.
-
-
67) Monascus purpureus is a yeast used commercially in the production of:
-
Citric acid
-
Blood cholesterol lowering statins
-
Ethanol
-
Streptokinase for removing clots from blood vessels
-
-
68) In the five-kingdom classification, Chlamydomonas and Chlorella have been included in
-
Monera
-
Protista
-
Algae
-
Plantae
-
-
69) Identify the likely organisms (a), (b) (c) and (d) in the food web shown below
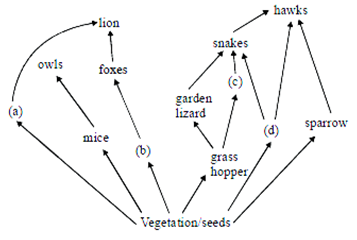
-
-
70) A test cross is carried out to
-
Determine whether two species or varieties will breed successfully
-
Determine the genotype of a plant at F2
-
Predict whether two traits are linked
-
Assess the number of alleles of a gene
-
-
71) Read the following five statements (A – D) and answer as asked next to them.
- In Equisetum the female gametophyte is retained on the parent sporophyte.
- In Ginkgo male gametophyte is not independent.
- The sporophyte in Riccia is more developed than that in Polytrichum.
- Sexual reproduction in Volvox is isogamous.
- The spores of slime molds lack cell walls.
How many of the above statements are correct?
-
One
-
Two
-
Three
-
Four
-
72) Which one of the following human organs is often called the “graveyard” of RBCs?
-
Liver
-
Gall bladder
-
Kidney
-
Spleen
-
-
73) Which one of the following elements in plants is not remobilised?
-
Sulphur
-
Phosphorus
-
Calcium
-
Potassium
-
-
74) A drupe develops in
-
Tomato
-
Mango
-
Wheat
-
Pea
-
-
75) Ground tissue includes
-
All tissues internal to endodermis
-
All tissues external to endodermis
-
All tissues except epidermis and vascular bundles
-
Epidermis and cortex
-
-
76) In land plants the guard cells differ from other epidermal cells in having
-
Chloroplasts
-
Cytoskeleton
-
Mitochondria
-
Endoplasmic reticulum
-
-
77) The ovary is half inferior in flowers of
-
Guava
-
Peach
-
Cucumber
-
Cotton
-
-
78) The cork cambium, cork and secondary cortex are collectively called
-
Phellem
-
Phelloderm
-
Phellogen
-
Periderm
-
-
79) Which one of the following is wrongly matched?
-
Cassia – Imbricate aestivation
-
Root pressure – Guttation
-
Puccinia – Smut
-
Root – Exarch protoxylem
-
-
80) Flowers are zygomorphic in
-
Datura
-
Mustard
-
Gulmohur
-
Tomato
-
-
81) CAM helps the plants in
-
Reproduction
-
Conserving water
-
Secondary growth
-
Disease resistance
-
-
82) Of the total incident solar radiation the proportion of PAR is
-
More than 80%
-
About 70%
-
About 60%
-
Less than 50%
-
-
83) A prokaryotic autotrophic nitrogen fixing symbiont found in
-
Pisum
-
Alnus
-
Cycas
-
Cicer
-
-
84) Nucellar polyembryony is reported in species of
-
Brassica
-
Citrus
-
Gossypium
-
Triticum
-
-
85) Filiform apparatus is a characteristic feature of
-
Zygote
-
Suspensor
-
Egg
-
Synergid
-
-
86) What would be the number of chromosomes of the aleurone cells of a plant with 42 chromosomes in its root tip cells?
-
21
-
42
-
63
-
84
-
-
87) Wind pollination is common in
-
Orchids
-
Legumes
-
Lilies
-
Grasses
-
-
88) In which one of the following pollination is autogamous?
-
Cleistogamy
-
Geitonogamy
-
Xenogamy
-
Chasmogamy
-
-
89) Mass of living matter at a trophic level in an area at any time is called
-
Standing state
-
Standing crop
-
Detritus
-
Humus
-
-
90) Which one of the following statements is wrong in case of Bhopal tragedy?
-
It took place in the night of December 2/3, 1984.
-
Methyl isocyanate gas leakage took place.
-
Thousands of human beings died.
-
Radioactive fallout engulfed Bhopal.
-
-
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
-
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
-
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
-
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
-
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
-
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- 83
- 84
- 85
- 86
- 87
- 88
- 89
- 90