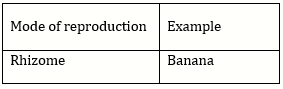
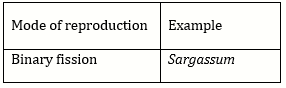
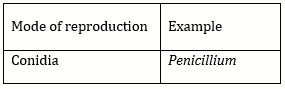
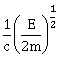Question Paper (Section wise)
-
1) A carbon resistor of (47 ± 4.7) kΩ is to be marked with rings of different colours for its indentification. The colour code sequence will be
-
Yellow-Green - Violet-Gold
-
Yellow-Violet-Orange-Silver
-
Violet-Yellow-Orange-Silver
-
Green-Orange -Violet -Gold
-
-
2) A set of 'n' equal resistors, of value 'R' each, are connected in series to a battery of emf 'E' and internal resistance 'R'. The current drawn is I. Now, the 'n' resistors are connected in parallel to the same battery. Then the current drawn from battery becomes 10 I. The value of 'n' is
-
20
-
11
-
10
-
9
-
-
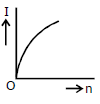
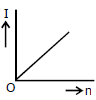
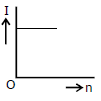
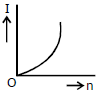
3) A battery consists of a variable number 'n' of identical cells (having internal resistance 'r' each) which are connected in series. The terminals of the battery are short-circuited and the current I is measured. Which of the graphs shows the correct relationship between I and n?
-
-
4) A body initially at rest and sliding along a frictionless track from a height h (as shown in the figure) just completes a vertical circle of diameter AB = D. The height h is equal to
-
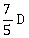
-
D
-
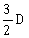
-
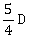
-
-
5) Three objects, A: (a solid sphere), B: (a thin circular disk) and C: (a circular ring), each have the same mass M and radius R. They all spin with the same angular speed ω about their own symmetry axes. The amounts of work (W) required to bring them to rest, would satisfy the relation.
-
WB > WA > WC
-
WA > WB > WC
-
WC > WB > WA
-
WA > WC > WB
-
-
6) Which one of the following statements is incorrect?
-
Friction force opposes the relative motion
-
Limiting value of static friction is directly proportional to normal reaction
-
Rolling friction is smaller than sliding friction
-
Coefficient of sliding friction has dimensions of length
-
-
7) A moving block having mass m, collides with another stationary block having mass 4m. The lighter block comes to rest after collision. When the initial velocity of the lighter block is v, then the value of coefficient of restitution (e) will be
-
0.8
-
0.25
-
0.5
-
0.4
-
-
8) A tuning fork is used to produce resonance in a glass tube. The length of the air column in this tube can be adjusted by a variable piston. At room temperature of 27°C two successive resonances are produced at 20 cm and 73 cm of column length. If the frequency of the tuning fork is 320 Hz, the velocity of sound in air at 27°C is
-
350 m/s
-
339 m/s
-
330 m/s
-
300 m/s
-
-
9) The electrostatic force between the metal plates of an isolated parallel plate capacitor C having a charge Q and area A is
-
proportional to the square root of the distance between the plates.
-
linearly proportional to the distance between the plates.
-
independent of the distance between the plates.
-
inversely proportional to the distance between the plates.
-
-
10) If θ1 and θ2 be the apparent angles of dip observed in two vertical planes at right angles to each other, then the true angle of dip θ is given by:
-
tan2ϕ = tan2 ϕ 1 + tan2 ϕ 2
-
cot2 ϕ = cot2 ϕ1 - cot2 ϕ 2
-
tan2ϕ = tan2 ϕ1 - tan2 ϕ 2
-
cot2 ϕ = cot2 ϕ 1 + cot2 ϕ 2
-
-
11) Two cars moving in opposite directions approach each with speed of 22 m/s and 16.5 m/s respectively. The driver of the first car blows a horn having a frequency 400 Hz. The frequency heard by the driver of the second car is [velocity of sound 340 m/s]
-
361 Hz
-
411 Hz
-
448 Hz
-
350 Hz
-
-
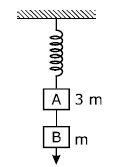
12) Two blocks A and B of masses 3m and m respectively are connected by a massless and inextensible string. The whole system is suspended by a massless spring as shown in figure. The magnitudes of acceleration of A and B immediately after the string is cut, are respectively.
-
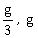
-
g, g
-
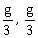
-
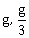
-
-
13) A thin prism having refracting angle 10° is made glass of refracting index 1.42. This prism is combination with another thin prism of glass of refracting index 1.7. This combination produces dispersion without deviation. The refracting angle of second prism should be:
-
6°
-
8°
-
10°
-
4°
-
-
14) The acceleration due to gravity at a height 1 km above the earth is the same as at a depth d below the surface of earth. Then:
-
d = 1km
-
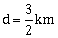
-
d= 2 km
-
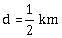
-
-
15) A potentiometer is an accurate and versatile device to make electrical measurements of E. M. F. because the method involves:
-
potential gradients
-
a condition of no current flow through the galvanometer
-
a combination of cells, galvanometer and resistance
-
Cells
-
-
16) A spherical black body with a radius of 12 cm radiates 450 watt power at 500 K. If the radius were halved and the temperature doubled, the power radiated in watt would be:
-
450
-
1000
-
1800
-
225
-
-
17) Figure shows a circuit that contains three identical resistor with resistance R = 9.0 Ω each, two identical inductors with inductance L = 2.0 mH each, and an ideal battery with emf ε = 18 V. The current “i” through the battery just after the switch closed is,…
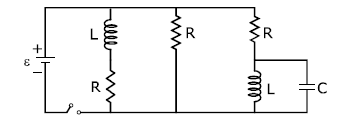
-
0.2 A
-
2 A
-
4 A
-
2 mA
-
-
18) Radiaactive material ‘A’ as decay constant ‘8 λ’ and material ‘B’ has decay constant ‘λ’. Initially they have same number of nuclei. After what time, the ratio of number of muclei of material ‘B’ to that ‘A’ will be
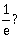
-
-
19) A light rod of length l has two masses m1 and m2 attached to its two ends. The moment of inertia of the system about an axis perpendicular of the rod and passing through the center of mass is
-
-
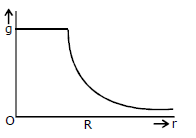
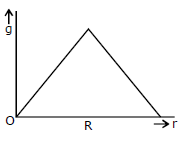
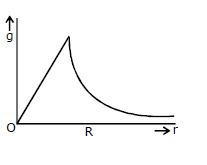
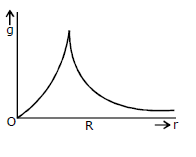
20) Starting from the centre of the each having radius R, the variation of g (acceleration due to gravity) is shown by
-
-
21) A satellite of mass m is orbiting the earth (or radius R) at a height h from its surface. The total energy of the satellite in term of g0. The value of acceleration due to gravity at the earth’s surface, is
-
-
22) A rectangular film of liquid is extended from (4cm × 2 cm) to (5 cm × 4 cm). if the work done is 3 × 10-4 J, the value of the surface tension of the liquid is
-
8.0 N m-1
-
0.250 N m-1
-
0.125 N m-1
-
0.2 N m-1
-
-
23) Three liquids of densities ρ1 ρ 2 and ρ 3 (with ρ 1 > ρ 2 > ρ 3), having the same value of surface T, rise to the same height in three identical capillaries. The angles of contact θ1, θ2 and θ3 obey
-
-
24) Two identical bodies are made of a material for which the heat capacity increase with temperature. One of these is at 100°C, while the other one is at 0°C. if the two bodies are brought into contact, then, assuming no heat loss, the final common temperature is
-
0°C
-
50°C
-
more than 50°C
-
less than 50°C but greater than 0°C
-
-
25) A body cools from a temperature 3T to 2T in 10 minutes. The room temperature is T. Assume that Newton’s law of cooling is applicable. The temperature of the body at the end of next 10 minutes will be
-
T
-
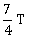
-
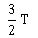
-
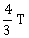
-
-
26) One mole of an ideal monatomic gas undergoes a process described by the equation PV3 = constant. The heat capacity of the gas during this process is
-
R
-
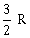
-
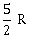
-
2 R
-
-
27) The temperature inside a refrigerator is t2 °C and the room temperature is t1 °C. The amount of heat delivered to the room for each joule of electrical energy consumed ideally will be
-
-
28) A uniform circular disc of radius 50 cm at rest is free to turn about an axis which is perpendicular to its plane and passes through its centre. It is subjected to a torque which produces a constant angular acceleration of 2.0 rad s-2. Its net acceleration in ms-2 at the end of 2.0 s is approximately:
-
8.0
-
7.0
-
6.0
-
3.0
-
-
29) An electron of mass m and a photon have same energy E. The ratio of de-Broglie wavelengths associated with them is:
-
-
30) A disk and a sphere of same radius but different masses roll off on two inclined planes of the same altitude and length. Which one of the two objects gets to the bottom of the plane first?
-
Disk
-
Sphere
-
Both reach at the same time
-
Depends on their masses
-
-
31) The angle of incidence for a ray of light at a refracting surface of a prism is 45°. The angle of prism is 60°. If the ray suffers minimum deviation through the prism, the angle of minimum deviation and refractive index of the material of the prism respectively, are:
-
-
32) When an α–particle of mass ‘m’ moving with velocity ‘ν’ bombards on a heavy nucleus of charge ‘Ze’ its distance of closest approach from the nucleus depends on m as:
-
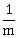
-
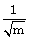
-
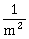
-
m
-
-
33) A particle of mass 10 g moves along a circle of radius 6.4 cm with a constant tangential acceleration. What is the magnitude of this acceleration if the kinetic energy of the particle becomes equal to 8×10-4 J by the end of the second revolution after the beginning of the motion?
-
0.1 m/s2
-
0.15 m/s2
-
0.18 m/s2
-
0.2 m/s2
-
-
34) The molecules of a given mass of a gas have r.m.s. velocity of 200 ms-1 at 27°C and 1.0 × 105 Nm-2 pressure. When the temperature and pressure of the gas are respectively, 127°C and 0.05 × 105Nm-2, the r.m.s. velocity of its molecules in ms-1 is:
-
-
35) A long straight wire of radius a carries a steady current I. The current is uniformly distributed over its cross–section. The ratio of the magnetic fields B and B’, at radial distances
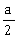 and 2a respectively, from the axis of the wire is
and 2a respectively, from the axis of the wire is-
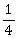
-
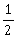
-
1
-
4
-
-
36) 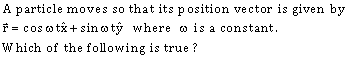
-
-
37) A parallel plate air capacitor has capacity ‘C’, distance of separation between paltes is ‘d’ and potential difference ‘V’ is applied between the plates. Force of attraction between the plates of the parallel plate air capacitor is-
-
-
38) The position vector of a particle
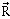 as a function of time is given by-
as a function of time is given by-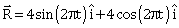 Where R is in meters, t is in second and
Where R is in meters, t is in second and 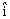 and
and 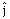 denote unit vectors along x and y direction, respectively. Which one of the following statements for the motion of particle?
denote unit vectors along x and y direction, respectively. Which one of the following statements for the motion of particle?-
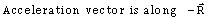
-
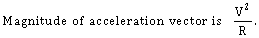
-
Magnitude of the velocity of particle is 8 meter/second
-
Path of the particle is circle of radius 3 meter.
-
-
39) A series R-C circuit is connected to an alternating voltage source. Consider two situations:
(i) When capacitor is air filled
(ii) When capacitor is mica filled
Current through resistor is I and voltage across capacitor is V then:
-
Va < Vb
-
Va > Vb
-
ia > ib
-
Va = Vb
-
-
40) A string is stretched between fixed points separated by 75.0 cm. It is observed to have resonant frequencies of 420 Hz and 315 Hz. There are no other resonant frequencies between these two. The lowest resonant frequency for this string is-
-
155 Hz
-
205 Hz
-
10.5 Hz
-
105 Hz
-
-
41) The coefficient of performance of a refrigerator is 5. If the temperature inside freezer is - 20°C, the temperature of the surrounding to which it rejects is
-
31°C
-
41°C
-
11°C
-
21°C
-
-
42) A photoelectric surface is illuminated successively by monochromatic light of wavelenght λ and π/2. If the maximum kinetic energy of the emitted photoelectrons in the second case is 3 times that in the first case, the work function of the surface of the material is-
-
-
43) In an astronomical telescope in normal adjustment a straight block line of length L is drawn on inside part of objective lens. The eye-piece from a real image of this line. The length of this image is I. The magnification of the telescope is:
-
-
44) Two slits in Young’s experiment have width in the ratio 1:25. The ratio of the intensity at the maxima and minima in the interference pattern
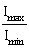 is:
is: -
-
45) Two vessels separately contain two ideal gases A and B at the same temperature, the pressure of A being twice that of B. Under such conditions, the density of A is found to be 1.5 time the density of B. The ratio of molecular weight of A and B is-
-
2/3
-
3/4
-
2
-
1/2
-
-
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
-
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
-
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
-
1) Which one of the following conditions will favour maximum formation of the product in the reaction:
A2 (g) + B2(g) ⇌ X2(g) ΔH =-X kJ
-
High temperature and high pressure
-
Low temperature and low pressure
-
Low temperature and high pressure
-
High temperature and low pressure
-
-
2) The correction factor ‘a’ to the ideal gas equation corresponds to:
-
electric field present between the gas molecules
-
volume of the gas molecules
-
density of the gas molecules
-
forces of attraction between the gas molecules
-
-
3) When initial concentration of the reactant is doubled, the half-life period of a zero order reaction:
-
is tripled
-
is doubled
-
is halved
-
remains unchanged
-
-
4) The bond dissociation energies of X2, Y2 and XY are in the ratio of 1:0.5:1. ΔH for the formation of XY is -200 kJ mol-1. The bond dissociation energy of X2 will be:
-
800 kJ mol-1
-
100 kJ mol-1
-
200 kJ mol-1
-
400 kJ mol-1
-
-
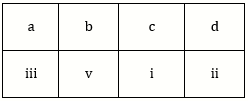
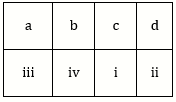
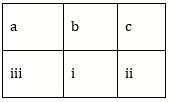
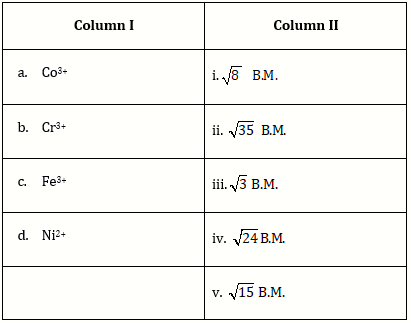
5) Match the metal ions given in column I with the spin magnetic moments of the ions given in Column II and assign the correct code:
-
-
6) Which one of the following ions exhibits d-d transition and paramagnetism as well?
-
-
7) The type of isomerism shown by the complex [CoCl2 (en)2] is
-
Ionization isomerism
-
Coordination isomerism
-
Geometrical isomerism
-
Linkage isomerism
-
-
8) The geometry and magnetic behavior of the complex [Ni(CO)4] are
-
square planar geometry and paramagnetic
-
tetrahedral geometry and diamagnetic
-
square planar geometry and diamagnetic
-
tetrahedral geometry and paramagnetic
-
-
9) Iron carbonyl. Fe (CO)5 is
-
trinuclear
-
mononuclear
-
tetranuclear
-
dinuclear
-
-
10) Which of the following pairs of compound is isoelectronic and isostructual?
-
TeI2, XeF2
-
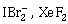
-
IF3, XeF2
-
BeCl2, XeF2
-
-
11) The IUPAC name of the compound
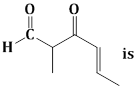
-
5–formylhex–2–en–3 one
-
5–methyl–4 oxohex–2–en–5–enal
-
3–keto–2–methylhex–5–enal
-
3–keto–2–methylhex–4–enal
-
-
12) Which one is the wrong statement?
-
The uncertainty principle is ΔE × Δt ≥ h/ 4π
-
Half filled and fully filled orbitals have greater stability due to greater exchange energy, greater symmetry and more balanced arrangement
-
The energy of 2s orbital is less than the energy of 2p orbital in case of hydrogen like atoms.
-
De-Brogle’s wavelength is given by
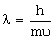 , where m = mass of the particle, υ = group velocity of the particle.
, where m = mass of the particle, υ = group velocity of the particle.
-
-
13) Which is the incorrect statement?
-
Density decrease in case of crystal with Schottky’s defect.
-
NaCl(s) insulator, silicon is semiconductor, silver is conductor, quartz is piezo electric crystal.
-
Frenkel defect is favoured in those ionic copounds in which sizes of cation and anions are almost equal.
-
FeO0.98 has non stoichiometric metal
-
-
14) The species, having bond angles of 120° is
-
ClF3
-
NCl3
-
BCl3
-
PH3
-
-
15) For a given reaction, ΔH = 35.5 kJ mol-1 and ΔS = 83.6 JK-1mol-1. The reaction is spontaneous at: (Assume that ΔH and ΔS do not vary with temperature)
-
T > 425 K
-
all temperatures
-
T > 298 K
-
T < 425 K
-
-
16) Which of the following is a sink for CO?
-
Microorganism present in the soil
-
Oceans
-
Plants
-
Haemoglobin
-
-
17) If molality of the dilute solution is doubled the value of molal depression constant (Kf) will be:
-
halved
-
tripled
-
unchanged
-
doubled
-
-
18) Which of the following is dependent on temperature?
-
Molarity
-
Mole fraction
-
Weight percentage
-
Molality
-
-
19) The solubility of AgCl(s) with solubility product 1.6 ×10–10 in 0.1 M NaCl solution would be
-
zero
-
1.26 × 10–5 M
-
1.6 × 10–9 M
-
1.6 × 10–11 M
-
-
20) Suppose the elements X and Y combine to form two compounds XY2 and X3Y2. When 0.1 mole of XY2 weights 10 g and 0.05 mole of X3Y2 weight 9 g, the atomic weights of X and Y are
-
30, 20
-
40, 30
-
60, 40
-
20, 30
-
-
21) The number of electrons delivered at the cathode during electrolysis by a current of 1 ampere in 60 seconds is (Charge on electron = 1.60 × 10–19 C)
-
7.48 × 1023
-
6 × 1023
-
6 × 1020
-
3.75 × 1020
-
-
22) Boric acid is an acid because its molecule
-
combines with proton from water molecule
-
contains replaceable H+ ion
-
gives up a proton
-
accepts OH– from water releasing proton
-
-
23) AlF3 is soluble in HF only in presence of KF. It is due to the formation of
-
K [AlF3H]
-
K3 [AlF3H3]
-
K3[AlF6]
-
AlH3
-
-
24) Zinc can be coated on iron to produce galvanized iron but the reverse is not possible. It is because
-
zinc has higher negative electrode potential than iron
-
zinc is lighter than iron
-
zinc has lower negative electrode potential than iron
-
zinc has lower negative electrode potential than iron
-
-
25) The suspension of slaked lime in water is known as
-
aqueous solution of slaked lime
-
limewater
-
quicklime
-
milk of lime
-
-
26) The hybridizations of atomic orbitals of nitrogen in
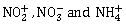 respectively are
respectively are-
sp2, sp and sp3
-
sp, sp3 and sp2
-
sp2, sp3 and sp
-
sp, sp2 and sp3
-
-
27) Which of the following fluoro-compounds is most likely to behave as a Lewis base?
-
SiF4
-
BF4
-
PF3
-
CF4
-
-
28) The correct thermodynamic conditions for the spontaneous reaction at all temperature is:
-
ΔH < 0 and ΔS = 0
-
ΔH > 0 and ΔS < 0
-
ΔH < 0 and ΔS > 0
-
ΔH < 0 and ΔS < 0
-
-
29) Lithium has a bcc structure. Its density is 530 kgm-3 and its atomic mass is 6.94 gmol-1 Calculate the edge length of a unit cell of Lithium metal. (NA = 6.02 × 1023 mol-1)
-
154 pm
-
352 pm
-
527 pm
-
264 pm
-
-
30) Which one of the following orders is correct for the bond dissociation enthalpy of halogen molecules?
-
I2 > Br2 > CI2 > F2
-
CI2 > Br2 > F2 > I2
-
Br2 > I2 > F2 > CI2
-
F2 > CI2 > Br2 > I2
-
-
31) Which of the following is an analgesic?
-
Novalgin
-
Penicillin
-
Streptomycin
-
Chloromycetin
-
-
32) Equal moles of hydrogen and oxygen gases are placed in a container with a pin-hole through which both can escape. What fraction of the oxygen escapes in the time required for one-half of the hydrogen to escape?
-
1/8
-
1/4
-
3/8
-
1/2
-
-
33) Consider the nitration of benzene using mixed conc. H2 SO4 and HNO3. If a larger amount of KHSO4 is added to the mixture, the rate of nitration will be:
-
faster
-
slower
-
unchanged
-
doubled
-
-
34) Predict the correct order among the following:
-
lone pair – lone pair > lone pair – bond pair > bond pair – bond pair
-
lone pair – lone pair > bond pair – bond pair > lone pair – bond pair
-
bond pair – bond pair > lone pair – bond pair > lone pair – lone pair
-
lone pair – bond pair > bond pair – bond pair > lone pair – lone pair
-
-
35) The product obtained as a results of a reaction of nitrogen with CaC2 is:
-
Ca(CN)2
-
CaCN
-
Ca(CN)2
-
Ca2CN
-
-
36) Consider the following liquid – vapour equilibrium.
Liquid ⇌ Vapour
Which of the following relations is correct?
-
-
37) The sum of coordination number and oxidation number of the metal M in the complex [M(en)2(C2O4)] Cl] is: ( where en is ethylenediamine)
-
8
-
9
-
6
-
7
-
-
38) Which of the statements given below is incorrect?
-
OF2 is an oxide of fluorine
-
Cl2O7 is an anhydride of perchloric acid
-
O3 molecules is bent
-
ONF is isoelectronic with O2N-
-
-
39) In the extraction of copper from its sulphide ore, the metal is finally obtained by the reduction of cuprous oxide with:
-
Sulphur dioxide
-
iron II sulphide
-
carbon monoxide
-
copper I sulphide
-
-
40) Which one of the following pairs of solution is not an acidic buffer?
-
H3PO4 and Na3PO4
-
HClO4 and NaClO4
-
CH3COOH and CH3COONa
-
H2CO3 and Na2CO3
-
-
41) Assuming complete ionization, same moles of which of the following compounds will require the least amount of acidified KMnO4 for complete oxidation?
-
FeNO2
-
FeSO4
-
FeSO3
-
FeC2O4
-
-
42) The number of structural isomers possible from the molecular formula C3H9N is:
-
3
-
4
-
5
-
2
-
-
43) 20.0 of a magnesium carbonate sample decomposes on heating to give carbon dioxide and 8.0 g magnesium oxide. What will be the percentage purity of magnesium carbonate in the sample?
-
84
-
75
-
96
-
60
-
-
44) Two possible stereo-structures of CH3CHOH. COOH, which are optically active, are called:
-
Mesomers
-
Diastereomers
-
Atropisomers
-
Enantiomers
-
-
45) The heat of combustion of carbon to CO2 is −393.5 kJ/mol. The heat release upon formation of 35.2 g of CO2 from carbon and oxygen gas is:
-
−3.15 kJ
-
−315 kJ
-
+315 kJ
-
−630 kJ
-
-
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
-
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
-
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
-
1) Which one is wrongly matched?
-
Gemma cups - Marchantia
-
Biflagellate zoospores - Brown algae
-
Uniflagellate gametes - Polysiphonia
-
Unicellular organism - Chlorella
-
-
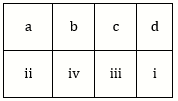
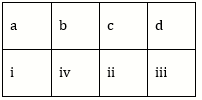
2) Match the items given in column I with those in column II and select the option given below:
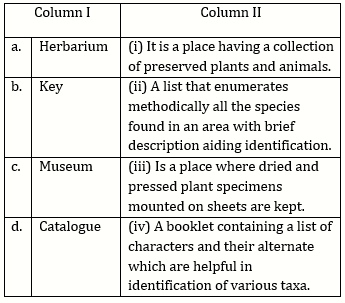
-
-
3) Which of the following flowers only once in its life-time?
-
Mango
-
Jackfruit
-
Bamboo species
-
Papaya
-
-
4) Which of the following pairs is wrongly matched?
-
XO type sex determination - Grasshopper
-
ABO blood grouping - Co-dominance
-
Starch synthesis in pea - Multiple alleles
-
T.H. Morgan - Linkage
-
-
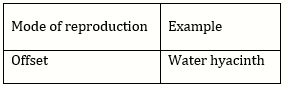
5) Offsets are produced by
-
Parthenocarpy
-
Mitotic divisions
-
Meiotic divisions
-
Parthenogenesis
-
-
6) Which of the following has proved helpful in preserving pollen as fossils?
-
Oil content
-
Cellulosic intine
-
Pollenkitt
-
Sporopollenin
-
-
7) Select the correct statement:
-
Spliceosomes take part in translation.
-
Punnett square was developed by a British scientist.
-
Franklin Stahl coined the term “linkage”.
-
Transduction was discovered by S. Altman.
-
-
8) The experimental proof for semiconservative replication of DNA was first shown in a
-
Plant
-
Bacterium
-
Fungus
-
Virus
-
-
9) Select the correct match:
-
Matthew Meselson and F. Stahl - Pisum sativum
-
Alfred Hershey and Martha Chase - TMV
-
Alec Jeffreys - Streptococcus pneumoniae
-
Francois Jacob and Jacques Monod - Lac operon
-
-
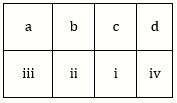
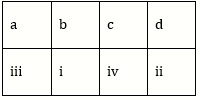
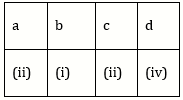
10) Match the items given in Column I with those in Column II and select the correct option given below:
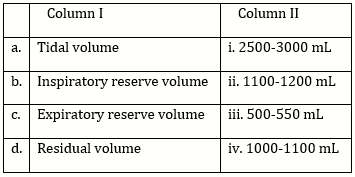
-
-
11) Which of the following options correctly represents the lung conditions in asthma and emphysema respectively?
-
Increased respiratory surface; Inflammation of bronchioles
-
Increased number of bronchioles; Increased respiratory surface
-
Inflammation of bronchioles; Decreased respiratory surface
-
Decreased respiratory surface; Inflammation a bronchioles
-
-
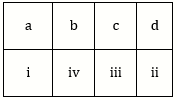
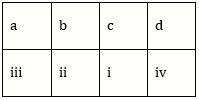
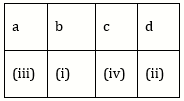
12) Match the items given in Column I with those in Column II and select the correct option given below:
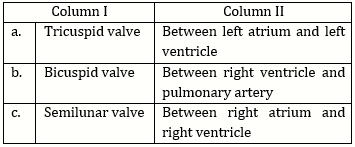
-
-
13) All of the following are part of an operon except
-
an enhancer
-
structural genes
-
an operator
-
a promoter
-
-
14) AGGTATCGCAT is a sequence from the coding strand of a gene. What will be the corresponding sequence of the transcribed mRNA?
-
ACCUAUGCGAU
-
UGGTUTCGCAT
-
AGGUAUCGCAU
-
UCCAUAGCGUA
-
-
15) According to Hugo de Vries, the mechanism of evolution is
-
Phenotypic variation
-
Saltation
-
Multiple step mutations
-
Minor mutations
-
-
16) Match the items given in column I with those in column II and select the correct option given below:
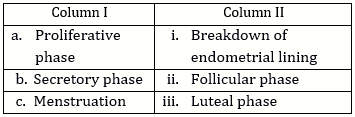
-
-
17) A woman has X-linked condition on one of her X chromosomes. This chromosome can be inherited by
-
Only grandchildren
-
Only sons
-
Only daughters
-
Both sons and daughters
-
-
18) Ciliates differ from all other protozoans in
-
Using pseudopodia for capturing prey
-
Having a contractile vacuole for removing excess water
-
Using flagella for locomotion
-
Having two types of nuclei
-
-
19) The final proof of DNA as the genetic material came from the experiments of:
-
Hershey and Chase
-
Avery, Mcleod and McCarty
-
Hargobind Khorana
-
Griffith
-
-
20) Among the following characters, which one was not considered by Mendel in his experiments on pea?
-
Trichomes-Glandular or non-glandular
-
Seed-Green or Yellow
-
Pod-Inflated or Constricted
-
Stem-Tall or Dwarf
-
-
21) Plants which produce characteristic pneumatophores and show vivipary belong to:
-
Halophytes
-
Psammophytes
-
Hydrophytes
-
Mesophytes
-
-
22) The pivot joint between atlas and axis is a type of:
-
Cartilaginous joint
-
Synovial joint
-
Saddle joint
-
Fibrous joint
-
-
23) With reference to factors affecting the rate of photosynthesis, which of the following statements is not correct?
-
Increasing atmospheric CO2 concentration upto 0.05% can enhance CO2 fixation rate.
-
C3 plants respond to higher temperature with enhanced photosynthesis while C4 plants have much lower temperature optimum.
-
Tomato is a greenhouse crop which can be grown in CO2- enriched atmosphere for higher yield.
-
Light saturation for CO2 fixation occurs at 10% of full sunlight.
-
-
24) DNA fragments are:
-
Negatively charged
-
Neutral
-
Either positively or negatively charged depending on their size
-
Positively charged
-
-
25) Which of the following components provides sticky character to the bacterial cell?
-
Nuclear membrane
-
Plasma membrane
-
Glycocalyx
-
Cell wall
-
-
26) Which of the following options best represents the enzyme composition of pancreatic juice?
-
Amylase, pepsin, trypsinogen, maltase
-
Peptidase, amylase, pepsin, rennin
-
Lipase, amylase, trypsinogen, procarboxypeptidase
-
Amylase, peptidase, trypsinogen, rennin
-
-
27) Which among these is the correct combination of aquatic mammals?
-
Dolphins, Seals, Trygon
-
Whales, Dolphins, Seals
-
Trygon, Whales, Seals
-
Seals, Dolphins, Sharks
-
-
28) Fruit and leaf drop at early stages can be prevented by the application of:
-
Ethylene
-
Auxins
-
Gibberellic acid
-
Cytokinins
-
-
29) Select the correct route for the passage of sperms in male frogs:
-
Testes → Vasa efferentia → Kidney → Seminal Vesicle → Urinogenital duct → Cloaca
-
Testes → Vasa efferentia → Bidder's canal → Ureter → Cloaca
-
Testes → Vasa efferentia → Kidney → Bidder's canal → Urinogenital duct → Cloaca
-
Testes → Bidder's canal → Kidney → Vasa efferentia → Urinogenital duct → Cloaca
-
-
30) In case of a couple where the male is having a very low sperm count, which technique will be suitable for fertilization?
-
Gamete intracytoplasmic fallopian transfer
-
Artificial insemination
-
Intracytoplasmic sperm injection
-
Intrauterine transfer
-
-
31) Which ecosystem has the maximum biomass?
-
Grassland ecosystem
-
Pond ecosystem
-
Lake ecosystem
-
Forest ecosystem
-
-
32) Lungs are made up of air-filled sacs, the alveoli. They do not collapse even after forceful expiration because of:
-
Inspiratory Reserve Volume
-
Tidal Volume
-
Expiratory Reserve Volume
-
Residual Volume
-
-
33) Presence of plants arranged into well-defined vertical layers depending on their height can be seen best in:
-
Tropical rain forest
-
Grassland
-
Temperate forest
-
Tropical savannah
-
-
34) Which of the following statements is correct?
-
The descending limb of loop of Henle is impermeable to water.
-
The ascending limb of loop of Henle is permeable to water.
-
The descending limb of loop of Henle is permeable to electrolytes.
-
The ascending limb of loop of Henle is impermeable to water.
-
-
35) Alexander Von Humboldt described for the first time:
-
Laws of limiting factor
-
Species area relationship
-
Population growth equation
-
Ecological biodiversity
-
-
36) Zygotic meiosis is characteristic of:
-
Fucus
-
Funaria
-
Chlamydomonas
-
Marchantia
-
-
37) In majority of angiosperms
-
A small central cell is present in the embryo sac.
-
Egg has a filiform apparatus.
-
There are numerous antipodal cells.
-
Reduction division occurs in the megaspore mother cells.
-
-
38) Pollination in water hyacinth and water lily is brought about by the agency of
-
Bats
-
Water
-
Insects or wind
-
Birds
-
-
39) The ovule of an angiosperm is technically equivalent to
-
Megaspore
-
Megasporangium
-
Megasporophyll
-
Megaspore mother cell
-
-
40) Taylor conducted the experiments to prove semiconservative mode of chromosome replication on
-
E. coli
-
Vinca rosea
-
Vida faba
-
Drosophila melanogaster
-
-
41) The mechanism that causes a gene to move from one linkage group to another is called
-
Crossing-over
-
Inversion
-
Duplication
-
Translocation
-
-
42) The equivalent of a structural gene is
-
Recon
-
Muton
-
Cistron
-
Operon
-
-
43) A true breeding plant is
-
Always homozygous recessive in its genetic constitution
-
One that is able to breed on its own
-
Produced due to cross-pollination among unrelated plants
-
Near homozygous and produces offspring of its own kind
-
-
44) Which of the following rRNAs acts as structure RNA as well as ribozyme in bacteria?
-
5.85S rRNA
-
5S rRNA
-
18S rRNA
-
23S rRNA
-
-
45) Stirred-tank bioreactors have been designed for
-
Ensuring anaerobic condition in the culture vessel
-
Purification of product
-
Addition of preservatives to the product
-
Availability of oxygen throughout the process
-
-
46) A molecule that can act as a genetic material must fulfill the traits given below, except
-
It should provide the scope for slow changes that are required for evolution.
-
It should be able to express itself in the form of ‘Mendelian characters’.
-
It should be able to generate its replica.
-
It should be unstable structurally and chemically.
-
-
47) DNA-dependent RNA polymerase catalyses transcription on one strand of the DNA which is called the
-
Antistrand
-
Template strand
-
Coding strand
-
Alpha strand
-
-
48) Interspecific hybridisation is the mating of
-
More closely related individuals within same breed for 4-6 generations
-
Animals within same breed with no common ancestors
-
Two different but related species
-
Superior males and females of different breeds
-
-
49) Which of the following is correct regarding AIDS causative agent HIV?
-
HIV does not escape but attacks the acquired immune response.
-
HIV is enveloped virus containing one molecule of single-stranded RNA and one molecule of reverse transcriptase.
-
HIV is enveloped virus that contains two identical molecules of single-stranded RNA and two molecules of reverse transcriptase.
-
HIV is unenveloped retrovirus.
-
-
50) Among the following edible fishes, which one is a marine fish having rich source of omega- 3 fatty acids?
-
Mackerel
-
Mystus
-
Mangur
-
Mrigala
-
-
51) Match Column-I with Column-II and selected the correct option using the codes given below:
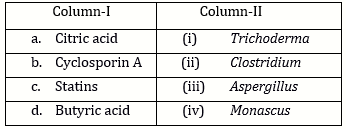
-
-
52) Biochemical Oxygen Demand (BOD) may not be a good index for pollution of water bodies receiving effluents from
-
Sugar industry
-
Domestic sewage
-
Dairy industry
-
Petroleum industry
-
-
53) The principle of competitive exclusion was stated by
-
Verhulst and Pearl
-
C. Danvin
-
G. F. Gause
-
MacArthur
-
-
54) Which of the following National Park is home to the famous musk deer of Hangul?
-
Dachigam National Park, Jammu & Kashmir
-
Keibul Lamjao National Park, Manipur
-
Bandhavgarh National Park, Madhya Pradesh
-
Eaglenest Wildlife Sanctuary, Arunachal Pradesh
-
-
55) Joint Forest Management concept was introduced in India during:
-
1960s
-
1970s
-
1980s
-
1990s
-
-
56) Pick out the correct statements:
(a) Haemophilia is a sex-linked recessive disease.
(b) Down’s syndrome is due to aneuploidy.
(c) Phenylketonuria is an autosomal recessive gene disorder.
(d) Sickle cell anaemia is an X-linked recessive gene disorder.
-
(a) and (d) are correct.
-
(b) and (d) are correct.
-
(a), (c) and (d) are correct.
-
(a), (b) and (c) are correct.
-
-
57) Which one of the following statements is wrong?
-
Cyanobacteria are also called blue-green algae.
-
Golden algae are also called desmids.
-
Eubacteria are also called false bacteria.
-
Phycomycetes are also called algal fungi.
-
-
58) Proximal end of the filament of stamen is attached to the:
-
Anther
-
Connective
-
Placenta
-
Thalamus or petal
-
-
59) Which of the following approaches does not give the defined action of contraceptive?
-
Barrier methods prevent fertilization.
-
Intra uterine devices increase phagocytosis of sperms, suppress sperm motility and fertilising capacity of sperms.
-
Hormonal contraceptives prevent/retard the entry of sperms, prevent ovulation and fertilization.
-
Vasectomy prevents spermatogenesis.
-
-
60) The Taq polymerase enzyme is obtained from:
-
Thermus aquaticus
-
Thiobacillus ferroxidans
-
Bacillus subtilis
-
Pseudomonas putida
-
-
61) Identify the correct statement on ‘inhibin’:
-
It inhibits the secretion of LH, FSH and prolactin.
-
It is produced by granulose cells in the ovary and inhibits the secretion of FSH.
-
It is produced by granulose cells in the ovary and inhibits the secretion pf LH.
-
It is produced by nurse cells in testes and inhibits the secretion of LH.
-
-
62) Which part of the tobacco plant is infected by Meloidogyne incognita?
-
Flower
-
Leaf
-
Stem
-
Root
-
-
63) Antivenom injection contains preformed antibodies while polio drops that are administered into the body contain:
-
Activated pathogens
-
Harvested antibodies
-
Gamma globulin
-
Attenuated pathogens
-
-
64) Which one of the following cell organelles is enclosed by a single membrane?
-
Mitochondria
-
Chloroplasts
-
Lysosomes
-
Nuclei
-
-
65) Lack of relaxation between successive stimuli in sustained muscle contraction is known as:
-
Spasm
-
Fatigue
-
Tetanus
-
Tonus
-
-
66) Which of the following is not a stem modification?
-
Pitcher of Nepenthes
-
Thorns of citrus
-
Tendrils of cucumber
-
Flattened structures of Opuntia
-
-
67) Water soluble pigments found in plant cell vacuoles are:
-
Xanthophylls
-
Chlorophylls
-
Carotenoids
-
Anthocyanins
-
-
68) Select the correct statement:
-
Gymnosperms are both homosporous and heterosporous.
-
Salvinia, Ginkgo and Pinus all are gymnosperms.
-
Sequoia in one of the tallest trees.
-
The leaves of gymnosperms are not well adapted to extremes of climate.
-
-
69) Which of the following is not required for any of the techniques of DNA fingerprinting available at present?
-
Polymerase chain reaction
-
Zinc finger analysis
-
Restriction enzymes
-
DNA-DNA hybridisation
-
-
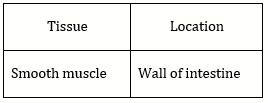
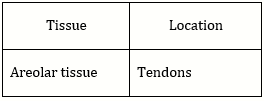
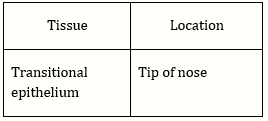
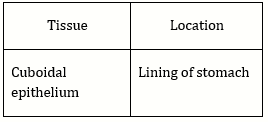
70) Which type of tissue correctly matches with its location?
-
-
71) A plant in your garden avoids photorespiratory losses, has improved water use efficiency, shows high rates of photosynthesis at high temperatures and has improved efficiency of nitrogen utilisation. In which of the following physiological groups would you assign this plant?
-
C3
-
C4
-
CAM
-
Nitrogen fixer
-
-
72) Which of the following structures is homologous to the wing of a bird?
-
Dorsal fin of shark
-
Wing of moth
-
Hindlimb of rabbit
-
Flipper of whale
-
-
73) Flowers are unisexual in:
-
Pea
-
Cucumber
-
China rose
-
Onion
-
-
74) Roots play insignificant role in absorption of water in:
-
Sunflower
-
Pistia
-
Pea
-
Wheat
-
-
75) Balbiani rings are sites of:
-
Lipid synthesis
-
Nucleotide synthesis
-
Polysaccharide synthesis
-
RNA and protein synthesis
-
-
76) Which of the following pairs is not correctly matched?
-
-
77) Ectopic pregnancies are referred to as:
-
Pregnancies with genetic abnormality.
-
Implantation of embryo at site other than uterus.
-
Implantation of defective embryo in the uterus.
-
Pregnancies terminated due to hormonal imbalance.
-
-
78) Choose the wrong statement:
-
Penicillium is multicelluar and produces antibiotics.
-
Neurospora is used in the study of biochemical genetics.
-
Morels and truffles are poisonous mushrooms.
-
Yeast is unicellular and useful in fermentation.
-
-
79) The function of gap junction is to:
-
Perform cementing to keep neighbouring cells together.
-
Facilitate communication between adjoining cells by connecting the cytoplasm for rapid transfer of ions, small molecules and some large molecules.
-
Separate two cells from each other.
-
Stop substance from leaking across a tissue.
-
-
80) Axile placentation is present in:
-
Dianthus
-
Lemon
-
Pea
-
Argemone
-
-
81) Which of the following are not membrane-bound?
-
Vacuoles
-
Ribosomes
-
Lysosomes
-
Mesosomes
-
-
82) In his classic experiments on pea plants, Mendel did not use:
-
Seed colour
-
Pod length
-
Seed shape
-
Flower position
-
-
83) During ecological succession:
-
The gradual and predictable change in species composition occurs in a given area.
-
The establishment of a new biotic community is very fast in its primary phase.
-
The number and types of animals remain constant.
-
The changes lead to a community that is in near equilibrium with the environment and is called pioneer community.
-
-
84) The body cells in cockroach discharge their nitrogenous waste in the haemolymph mainly in the form of
-
Ammonis
-
Potassium urate
-
Uric acid
-
Calcium carbonate
-
-
85) Which of the following biomolecules does have a phosphodiester bond?
-
Fatty acids in a diglyceride
-
Monosaccharides in a polysaccharide
-
Amino acids in a polypeptide
-
Nucleic acids in a nucleotide
-
-
86) The UN conference of Parties on climate change in the year 2012 was held at
-
Durban
-
Doha
-
Lima
-
Warsaw
-
-
87) Arrange the following events of meiosis in correct sequence:
a. Crossing over
b. Synapsis
c. Terminalisation of chiasmata
d. Disappearance of nucleolus
-
b, a, d, c
-
b, a, c, d
-
a, b, c, d
-
b, c, d, a
-
-
88) Root pressure develops due to:
-
Active absorption
-
Low osmotic potential in soil
-
Passive absorption
-
Increase in transpiration
-
-
89) Which one of the following animals has two separate circulatory pathways?
-
Frog
-
Lizard
-
Whale
-
Shark
-
-
90) Which of the following events is not associated with ovulation in human female?
-
Decrease in estradiol
-
Full development of Graafian follicle
-
Release of secondary oocyte
-
LH surge
-
-
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
-
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
-
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
-
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
-
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
-
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- 83
- 84
- 85
- 86
- 87
- 88
- 89
- 90